-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2014; 4(2): 23-28
doi:10.5923/j.cmd.20140402.02
Comparison of Some Urinary Biomarkers of Acute Kidney Injury in Term New Born with and without Asphyxia
Manal Abd El-Salam1, Manal Mohamed Zaher1, Ragaa Abd El-Salam Mohamed1, Layla Yossef Al Shall2, Rayyh A. M. Saleh2, Amal A. Hegazy3, 4
1Department of Pediatrics, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
2Department of Clinical pathology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
3Family and Community Medicine Department, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
4Community and Occupational Medicine Department, Faculty of Medicine (for girls), Al-Azhar University Cairo, Egypt
Correspondence to: Manal Abd El-Salam, Department of Pediatrics, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
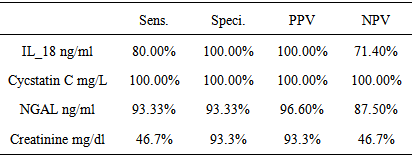
Background: Acute kidney injury (AKI) is a frequent clinical condition in neonatal intensive care unit (NICU). Urinary biomarkers can diagnose AKI within hours of an insult that has been discovered. The discovery of biomarkers for AKI that might enable early recognition and clinical intervention to limit renal injury. Objective: Was to evaluate urinary levels of cystatin C, Neutrophil gelatinase-associated lipocalin (NGAL) and IL -18 as early predictive biomarkers of acute kidney injury in term asphyxiated newborn.Patients and Methods: The study included 30 full terms newborn with asphyxia in the first 48 hours of delivery. They were selected from the NICU of Al –Zahraa hospital, Al-Azher University. Also the study included 30 healthy newborn ages and sex matched as a control group. Arterial blood gases (ABG), serum creatinine, urinary cystatin C, (uNGAL) and uIL- 18 were assessed among studied groups.Results: There was a significant increase in the serum creatinine level in the newborn with asphyxia than the control group, but both were in the normal reference range. There was a high significant decrease in pH and o2 saturation in term asphyxiated newborn than the control group. Asphyxiated neonates had significantly higher uCysC, uNGAL and uIL-18 compared to the controls. ucystatin C 100.00% sensitive and specific for prediction of AKI followed by IL-18 100.00% specific, 80.00% sensitivity and NGAL, 93.33% sensitive, 93.33% specific while sCr 46.7 % sensitive, 93.3% specific.Conclusion: uCysC, uNGAL and uIL-18 are specific and sensitive biomarkers for early AKI diagnosis in term asphyxiated newborn: urinary CysC, NGAL and IL18 have a significant predictive value for the identification of AKI inthe newborn with asphyxia as compared to SCr.
Keywords: Perinatal asphyxia, AKI, uCysC, uNGAL and u IL-18
Cite this paper: Manal Abd El-Salam, Manal Mohamed Zaher, Ragaa Abd El-Salam Mohamed, Layla Yossef Al Shall, Rayyh A. M. Saleh, Amal A. Hegazy, Comparison of Some Urinary Biomarkers of Acute Kidney Injury in Term New Born with and without Asphyxia, Clinical Medicine and Diagnostics, Vol. 4 No. 2, 2014, pp. 23-28. doi: 10.5923/j.cmd.20140402.02.
1. Introduction
- Perinatal asphyxia is the most common cause of AKI in newborns, which results mostly from impaired renal perfusion. As many as 61% of severely asphyxiated infants may develop AKI that is predominantly non-oliguric [1].In current clinical practice, acute kidney injury is typically diagnosed by measuring serum creatinine. Unfortunately; creatinine is an unreliable indicator of AKI [2]. Serum creatinine varies with age, sex, muscle bulk, metabolism, drugs and hydration status. It will not change until >50% of kidney function that has already been lost [3]. The estimated incidence of AKI in critically ill neonates is between 8% and 24% and mortality rates are between 10% and 61% [4]. It is unlikely that a single biomarker will satisfy all of necessary assessments. Thus, a panel of marker for foetal and neonatal assessment could predict patients at risk for developing severe kidney injury [5].Urine is an excellent source of biomarkers in kidney disease because it has been estimated that normally 70% of the urinary proteins originate from the kidney and the urinary tract [6]. These biomarkers will change our approach to the diagnosis AKI and, hopefully, lead to better preventative and therapeutic interventions which will improve outcomes of critically ill neonates [7]. Currently, the most promising early non-invasive biomarkers of AKI are serum and urinary neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), urinary interleukin-18(IL-18), and serum cystatin C [8].Cystatin C is a cysteine protease inhibitor that is synthesized and released into the blood at a relatively constant rate by all nucleated cells. It is freely filtered by the glomerulus, completely reabsorbed by the proximal tubule and not secreted [9]. In addition, fetal sCysC levels do not show any relationship to maternal sCysC levels [10].NGAL, known as Lipocain-2(lcn2) as well as Siderocalin, is an ubiquitos 25 kDa protein of 178 amino acids, covalently bound to gelatinize from human neutrophils [11].One in particular, NGAL was recently identified as one of the earliest and most robustly induced proteins by the kidney after ischemic injury. This is reflected by the rapid rise in urinary NGAL reported in AKI. NGAL concentration in the serum and urine has been demonstrated to be a sensitive and specific early marker of AKI after cardiac surgery [12].Urinary interleukin-18 is also being promoted as an early non-invasive biomarker of AKI. Interleukin-18 is an18-kDa pro-inflammatory cytokine initially synthesized in its inactive form (24-kDa) and subsequently cleaved by caspase-1 in the epithelial cells of the proximal tubules into its active form. It is easily detected in the urine following ischemic AKI in mice models [13]. Urinary IL-18 performed best as a predictor of AKI at 12 h compared to other time points. Both uNGAL and uIL-18 were independently associated with duration of AKI [14].The aim was to evaluate urinary levels of cystatin C, (NGAL) and IL 18 as an early predictive biomarkers of acute kidney injury in term asphyxiated newborn.
2. Subjects and Methods
- This is a cross sectional comparative; it was carried out in the NICU of AL-Zahraa hospital, Al-Azher University during the period from March 2013 to June 2013. The study included 30 full terms neonates with perinatal asphyxia. They were (12) females and (18) males. Also it included 30 ages and sexes matched newborn served as a control group. AKI biomarkers were compared between term asphyxiated neonates group and healthy controls. Full term newborns were considered to be exposed to perinatal asphyxia according to the criteria of American Academy of Pediatrics [15] which are (at least two of the following): Apgar score ≤3 at 1 minute or ≤ 5at 5 minute, Umbilical cord arterial (pH < 7.00) with base deficit >10 m mol/L. The presence of postnatal clinical complications attributed to perinatal asphyxia such as abnormal neurological signs (seizure, coma, hypotonia) and multiple organs involvement (kidney, lungs, liver, heart, intestine). Premature and full term newborn with: neonatal sepsis, multiple congenital anomalies, inborn error of metabolism and also newborn to the mother who suffered from chronic kidney disease were excluded from the study. Informed consent was obtained from the participating parents in adherence with the guidelines of the ethical committee of AL-Zahraa hospital, AL-Azher University, Cairo, Egypt. All studied children were be subjected to:1-Full history taking including:- Prenatal history including maternal condition during pregnancy (diseases or medications …).- Natal history including mode and duration of delivery, premature rupture of membrane).- Post natal history including 1st cry, cyanosis, sleepiness, respiratory distress, convulsion, APGAR score determination at one minute, 5 and 10 minutes.2-Clinical examination:Full general and local examination with stress on the following:- Gestational age assessment using New Ballard Score [16]- Full neurological examination within the first 24 hours.3-Laboratory Investigations:• Routine investigations: including (ABG & creatinine). Specific investigations including: Urinary levels of: Cystatin C – Neutrophil gelatinase-associated lipocalin (NGAL) and IL 18 by ELISA KIT.Sampling and procedure:Blood sampling: * 0.5ml was taken on heparenized syring from the cord blood at birth for abg (ABG)* 2 ml were taken in plain vacutainer for serum creatinine (in the 1st 48 hours of delivery).Urine samplingUrine samples were collected from the newborn with asphyxia in their first 48 hours of their admission to NICU using urine bags. Urine samples were centrifuged at 4000 rpm for 10 minutes. Urine supernatants were aliquoted and stored at −20°C for subsequent analysis of NGAL, cystatin C and IL-18. Using of ELISA immunoassay Kit according to the manufacture’s instructions (Glory Science, USA).NGAL assay:Samples are incubated in micro-plate wells pre-coated with polyclonal anti-human lipocalin-2 antibody. After one hour incubation and washing, biotin-labeled polyclonal anti-human lipocalin-2 antibody is added and incubated with captured lipocalin-2 for one hour. After another washing, streptavidin-HRP conjugate is added. After 30 minutes incubation and the last washing step, the remaining conjugate is allowed to react with the substrate solution (TMB). The reaction is stopped by addition of acidic solution and absorbance of the resulting yellow product is measured. The absorbance is proportional to the concentration of Lipocalin-2. A standard curve is constructed by plotting absorbance values against concentrations of Standards, and concentrations of unknown samples are determined using this standard curve. Assay range in serum, plasma and urine: 0.3- 10.0 ng/ml. detection limit: 0.02ng/ml (Stejskal et al., 2008) [17].Human CystatinC Assay Test principleAdd Cystatin C to monoclonal antibody Enzyme well which is pre-coated with Human Cystatin C monoclonal antibody, incubation; then, add Cystatin C antibodies labeled with biotin, and combined with Streptavidin-HRP to form immune complex; then carry out incubation and washing again to remove the uncombined enzyme. Then add Chromogen Solution A, B, the color of the liquid changes into the blue and at the effect of acid, the color finally becomes yellow. The chroma of color and the concenthumanion of the Human Substance Cystatin C of sample were positively correlated.It seems that reference values for freshly collected urine samples range from 0.03 to 0.18
 mg/L (Conti et al., 2006) [18].IL-18 Assay Principle of testAdd Interleukin-18 (IL-18) to monoclonal antibody Enzyme well which is pre-coated with Human Interleukin-18 (IL-18) monoclonal antibody, incubation; then, add Interleukin-18 (IL-18) antibodies labeled with biotin, and combined with Streptavidin-HRP to form immune complex; then carry out incubation and washing again to remove the uncombined enzyme. Then add Chromogen Solution A, B, the color of the liquid changes into the blue. And at the effect of acid, the color finally becomes yellow. The chroma of color and the concentration of the Human Interleukin 18(IL-18) of sample were positively correlated. The range of the kit is 0.5ng/L→ 100ng/L Sensitivity 2.68ng/mL (Parikh et al., 2005) [19].Statistical analysisThe data were revised, coded, and entered to the PC computer and analyzed using SPSS (version 16). Data were expressed descriptively as mean ± standard deviation (SD) for quantitative parametric data. Comparison between groups was done using the student t test for parametric data. The diagnostic performance of urinary cystatin C, NGAL, LI-18 and serum creatinine was evaluated using receiver operating characteristic curve (ROC) analysis.The P-value was considered significant as the following: • P > 0.05: • Non significant• P < 0.05: *Significant• p < 0.01: **Highly statistically significant
mg/L (Conti et al., 2006) [18].IL-18 Assay Principle of testAdd Interleukin-18 (IL-18) to monoclonal antibody Enzyme well which is pre-coated with Human Interleukin-18 (IL-18) monoclonal antibody, incubation; then, add Interleukin-18 (IL-18) antibodies labeled with biotin, and combined with Streptavidin-HRP to form immune complex; then carry out incubation and washing again to remove the uncombined enzyme. Then add Chromogen Solution A, B, the color of the liquid changes into the blue. And at the effect of acid, the color finally becomes yellow. The chroma of color and the concentration of the Human Interleukin 18(IL-18) of sample were positively correlated. The range of the kit is 0.5ng/L→ 100ng/L Sensitivity 2.68ng/mL (Parikh et al., 2005) [19].Statistical analysisThe data were revised, coded, and entered to the PC computer and analyzed using SPSS (version 16). Data were expressed descriptively as mean ± standard deviation (SD) for quantitative parametric data. Comparison between groups was done using the student t test for parametric data. The diagnostic performance of urinary cystatin C, NGAL, LI-18 and serum creatinine was evaluated using receiver operating characteristic curve (ROC) analysis.The P-value was considered significant as the following: • P > 0.05: • Non significant• P < 0.05: *Significant• p < 0.01: **Highly statistically significant3. Results
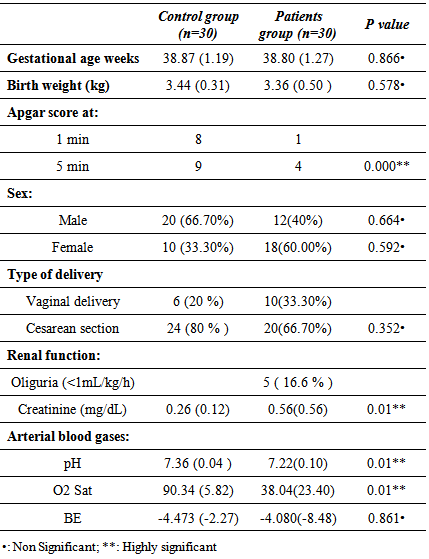
- Table 1 shows demographic, clinical characteristic and laboratory data of the studied groups; it revealed significant increase in the serum creatinine levels in patients group compared to the controls. Also it shows high significant decrease in pH and o2 sat in patients group than the control group.
|
|
|
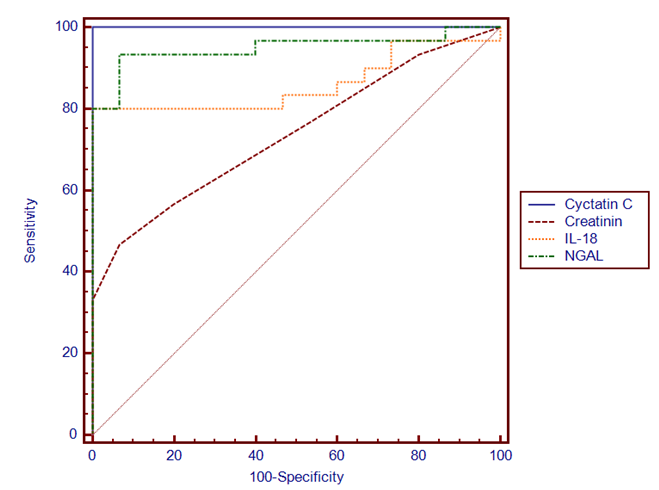 | Figure (1). Demonstrates specificity and sensitivity of the studied three urinary biomarkers and serum creatinine in term asphyxiated newborn |
4. Discussion
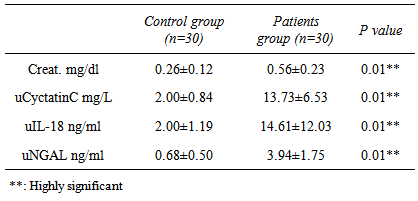
- Definitely, creatinine, as well as blood urea nitrogen (BUN) or urine markers of kidney injury (fractional excretion of sodium, urinary concentrating ability, casts), do not directly reflect cell injury, but rather the delayed functional consequences of the damage [20]. Existing studies on AKI biomarkers in neonates are limited and mainly concern reference values [21, 22]. After birth, neonatal SCr reflects maternal levels. Rather than maintaining a steady state, these levels then decline at varying rates over days to weeks depending on gestational age, such that changes (or lack of change) in SCr may be difficult to interpret when evaluating for AKI [23].In the present study we evaluated and compared three novel urine candidates AKI biomarkers (cystatin C, NGAL and IL18 in term neonates with acute perinatal asphyxia in their 1st 48 hours of delivery with controls. According to our results, asphyxiated neonates had significantly increased levels of uCysC which is normally filtered freely and completely reabsorbed and catabolized within the proximal tubule. Urine Cys C levels increase with structural and functional renal tubular impairment independent of glomerular filtration rate [24]. In agreement with our results [25], it’s reported that serum CysC levels were significantly higher in asphyxiated infants compared to control infants on day of life (DOL) 1, while urinary cystatin C (uCysC) was significantly higher in asphyxiated infants at all time points (DOL1, 3 and 10). Urinary CysC was also significantly higher in asphyxiated neonates with AKI compared to controls at all time points and in the asphyxiated group without AKI compared to controls on DOL 1 and 3. Serum CysC and uCysC showed favourable diagnostic performances as predictors of AKI on DOL 1. Also [26] found that infants with AKI had higher levels of uCysC compared to infants without AKI. We reported the diagnostic accuracy of uCysC with chosen cut of (3.4ng/L), sensitivity of 100%, specificity 100%.Treiber et al. [27] reported that the highest diagnostic accuracy was achieved with a chosen cut-off for cysC-umb of 1.67 mg/L (sensitivity of 84.0%, specificity of 90.0%) or 1.69 mg/L (sensitivity of 82.0%, specificity of 94.0%). The cut of point differences may be attributed to that uCysC rather than sCysC for AKI diagnosis was assessed as urine is an excellent source of biomarkers in kidney disease so the present study is another proof for that uCys C is an excellent biomarker for early AKI diagnosis. Additionally in the present study, asphyxiated neonates had significantly elevated uNGAL, as well as uIL18, these elevated urine biomarkers indicate that asphyxiated neonates do suffer acute tubular injury. However, the latter type of renal injury seems to occur in asphyxiated neonates irrespective of AKI development. Serum creatinine (Cr) is the most commonly used clinical measure of renal function, not injury, so it is a poor marker for diagnosis of AKI. SCr may not increase until about 25–50 % of renal function is lost [7] a fact which also highlights the insensitivity of the sCre-based AKI definitions, as in the present study the serum creatinin had poor diagnostic accuracy as (sensitivity of 46.7%, specificity 100%), therefore, “gold standard” sCre not only perplexes early documentation of AKI, but it lacks sensitivity for tubular injury making its use to define AKI problematic. The relative weakness of sCr in detecting this type of renal injury also becomes evident by the fact that asphyxiated neonates studied had higher blood and urine markers of tubular damage even on the 10th postnatal day, when sCre was already normalized [28] and so it is not surprising in the present to find that serum creatinine showed no significant differences among the studied groups.uCysC, and uNGAL as well as IL18 are of significant clinical importance as early predictors of post-asphyxia-AKI. Increased levels of these markers are supposed to indicate proximal tubular damage in the case of kidney injury [29]. In many studies on children (for example [30-32]) and adults (for example [33]), NGAL rises significantly in patients with AKI but not in controls. Furthermore, this rise in NGAL occurs at 24 to 48 h before the rise in SCr is. The better predictive ability of uIL-18 for the diagnosis of AKI in children compared to adults may be due to the release of IL-18 primarily through the kidney in children rather than the immune cells in adults [34], ROC analysis revealed that uNGAL, ucystatin c and IL18 at a cutoff value of 1.25 ng/mL, 3.4 ng/L, 3.75 ng/mL within the first 48 hours of life in asphyxiated neonates, can predict the development of AKI with high sensitivity and specificity. According to our results, asphyxiated neonates had significantly increased levels of u CysC, uNGAL and uIL-18 which are of significant clinical importance as early predictors of post-asphyxia-AKI. The strength of this study is the simultaneous evaluation for the first time of these three novel urinary renal injury biomarkers in asphyxiated neonates, in parallel to a control group of healthy neonates.
5. Conclusions
- Urinary CysC, NGAL and IL18 have a significant predictive value for the identification of AKI in the newborn with asphyxia. The combination of these three biomarkers may facilitate the early diagnosis of AKI as compared to SCr. Also this study, do suggest the possibility of early detection of renal damage in asphyxiated neonates that may subsequently be therapeutically targeted.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML