-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2013; 3(4): 92-100
doi:10.5923/j.cmd.20130304.04
Comparative Study of Circulating Cardiac Biomarker Galectin-3 and Troponin I in Heart Failure Patients
Hanan Mahmoud Fayed1, Mohamed Abdelrazek Alsenbesy2, Tahia Hashim Saleem3, Marwa Abd-elhady Ahmed2
1clinical and chemical pathology department- Qena faculty of medicine-South Valley University
2Internal Medicine department-Qena faculty of medicine- South Valley University
3Biochemistry department-Assuit faculty of medicine-Assuit University
Correspondence to: Hanan Mahmoud Fayed, clinical and chemical pathology department- Qena faculty of medicine-South Valley University.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
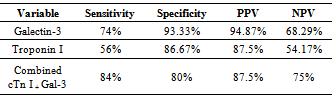
heart failure (HF) is a major growing health problem. Galectin-3 associated with fibrosis, inflammation and cardiac remodeling. There is increasing interest in the role of circulating cardiac troponin in detecting myocardial injury. So our aim was to determine serum values of Galectin-3 and troponin-I in hospitalized HF patients with different severity grades and correlate results with clinical, echocardiography in a cross sectional case-control study of fifty cases with HF from both sexes on standard medication. Thirty healthy volunteers’ subjects selected as controls. HF severity grade classified according to New York Heart Association; 8(16%) class II and 42(84%) class (III+IV). Left ventricular ejection fraction (LVEF) ≤ 40% in 23(46%) of HF. Compared to controls; Galectin-3 and troponin-I were significantly higher (18.40 ±11.5 ng/ml) vs (5.75 ±1.427 ng/ml); (p<0.001); (0.429± 0.630 ng/ml) vs (0.019±0.0544 ng/ml); (p= 0.004) respectively. Receiver operating characteristic (ROC) analysis for Galectin-3 showed the best cut off point which discriminates between disease and normal at Galectin-3 (≥7.36 ng/ml) with sensitivity 74%, specificity 96.7%, area under the curve (AUC) of 0.876 (p<0.0001), positive predictive value (PPV) 94.87% and negative predictive value (NPV) 68.29%. ROC analysis for troponin-I positivity shows 56% sensitivity, 86.67% specificity, PPV 87.5% and NPV 54.17%. Whereas combined[troponin-I positivity + Galectin-3] showed 84% sensitivity, 80 % specificity, PPV 87.5% and NPV 75% i.e. improve Galectin-3 sensitivity and the negative predicator value. So results support combined markers assessment in predicting severity in HF patients with preserved LVEF.
Keywords: Cardiac Biomarkers, Galectin-3, Heart Failure, Troponin I, Anemia, Echocardiography, Uric Acid, Fibrosis, Remodeling
Cite this paper: Hanan Mahmoud Fayed, Mohamed Abdelrazek Alsenbesy, Tahia Hashim Saleem, Marwa Abd-elhady Ahmed, Comparative Study of Circulating Cardiac Biomarker Galectin-3 and Troponin I in Heart Failure Patients, Clinical Medicine and Diagnostics, Vol. 3 No. 4, 2013, pp. 92-100. doi: 10.5923/j.cmd.20130304.04.
Article Outline
1. Introduction
- Heart failure (HF) is a clinical syndrome characterized by inadequate systemic perfusion to meet the body’s metabolic demands as a result of impaired cardiac function[1]. After the initial insult to the myocardium, HF is a disease with autonomic progression associated with ventricular dysfunction and cardiac remodeling[2]. The functional changes associated with HF can be systolic and/or diastolic in nature[3].HF has long been considered as irreversible and amenable only to palliative therapy. However, the idea of chronic HF as an irreversible end-stage process is challenged by experimental and clinical evidence of a possible improvement in the intrinsic defects of function and structure (afflicting the failing heart) following therapeutic intervention in the earliest phase of the cardiac alteration[4]. Early diagnosis of HF depends on the availability of specific, accurate and effective markers of disease[5]. A cardiovascular biomarker can be classified into different types, according to its pathophysiological characteristics and/or clinical use after the initial insult to the myocardium [6]. Galectin-3 (Gal-3) a macrophage-derived mediator that induces cardiac fibroblast proliferation, collagen deposition and ventricular dysfunction; thus it plays an important regulatory role in cardiac fibrosis and remodeling, which are key contributing mechanisms to the development and progression of HF[2]. Besides fibrosis, Gal-3 plays an important role in the inflammatory responses which are important players in the process of cardiac remodeling[7]. Gal-3 is specifically up regulated in decompensated HF compared with compensated HF in animal models; even before the onset of frank HF[8]. Moreover; Gal-3 up regulation may be a general phenomenon in left ventricular dysfunction[9].In HF, there is increased oxidative stress because of generation of reactive oxygen molecules and decrease in endogenous antioxidants which is responsible for endothelial dysfunction and progression of HF. Serum uric acid; a simple and readily available indirect biomarker of oxidative stress resulting from increased xanthine oxidase activity. It correlates with impaired hemodynamic and predicts adverse prognosis in heart failure[10].Cardiac troponin (cTn) complex involved in the regulation of cardiac muscle contraction; the fact that they are strictly intracellular proteins which are not found in the circulation of healthy individuals provides a high level of clinical sensitivity and specificity even when cardiac lesions are small. Thus, any significantly detectable troponin in circulation is considered a sign of acute myocardial cell damage. cTn in blood is the preferred biomarkers of myocardial injury; these are released as a result of severe ischemia and also as consequence of stress, injury secondary to increased inflammation, oxidative stress and neurohormonal activation[11]. Modest elevations of cTn levels are also found in patients with HF without ischemia[12]; and congestive HF[13]. The mechanism for this elevation is believed to be due to ongoing myocyte injury and progressive loss of cardiac myocytes; hence releasing cTn into the circulation[14].
2. The Aims of This Study were to
- 1) Determine the value of plasma Galectin-3 and Troponin I in HF patients.2) Assess some morbidity associated cofactors in HF patients such as hypernatraemia, hyperuricemia and anemia. 3) Correlate laboratory results with the clinical severity and the echocardiography findings.
3. Patients and Methods
- A cross sectional case/control study was conducted (after approval from medical ethics committee) on fifty patients (after obtaining informed consent) having the inclusion criteria from both sexes, who attended the Qena university hospital for one year. Inclusion criteria: Any patient with clinical manifestations of HF (either acute or chronic) with age above 18 years old who agree to participate in this study. Exclusion criteria: Patients's with associated with hepatic or renal diseases and refuse to participate in this study. HF severity grade was classified according to clinical symptoms by the New York Heart Association (NYHA)[15]. Echocardiogram performed to all patients for ischemia, hypertension or arrhythmia utilizing two-dimensional (2D) cardiovascular ultrasound system transthoracic (using Vivid 3, 2005, Germany, syncmaster450 MB) to assess cardiac chamber dimension, systolic and diastolic function, ejection fraction (Current practice uses two dimensional measurement to calculate LVEF with the Simpson’s Biplane method; tracing the endocardial border at end diastole and end systole to estimate left ventricle (LV) volumes and LVEF), regional wall motion abnormalities, valve heart disease, cardiomyopathies.A 30 healthy age and sex matched subjects selected as control. 10 ml venous blood drawn from all patients and controls then divided into:- • 5 ml in plain tube; centrifuged at 3,500 rpm for 15 min at 4 °C; the serum transferred into two of 1 ml cryotubes and stored at -80 °C for later analyses. • Rest of serum used for spectrophotometric measurements of serum urea, creatinine, uric acid, Sodium and albumin;[Cobas C311 (Roche diagnostics, Germany)].• Estimated glomerular filtration rate (eGFR); as an indicator of renal function estimated from serum creatinine using a formula that accounts for the influence of age on creatinine production, which was validated in patients with HF, and described in detail in modification of diet and renal disease (MDRD)[16].• 1.8 ml blood on citrate tubes for measurements of prothrombin time & concentration.• 3 ml blood on EDTA for Complete blood count "CBC" (Cell Dyn 1800-Abbott diagnostics).• Troponin I tested as instructed by the manufacturers using commercial automated chemiluminescent microparticle immune assay (CMIA) utilizing Chemi-Flex Technology (Architect i2000, Abbott diagnostics, USA). Normal value ≤0.30 ng/ml. The Architect STAT Troponin-I assay is a two-step immunoassay, in the first step, sample, assay diluent and anti-troponin-I antibody-coated paramagnetic microparticles (mouse, monoclonal) are combined. Troponin-I present in the sample binds to the anti-troponin-I coated microparticles. After incubation and wash, anti-troponin-I acridinium-labeled (mouse, monoclonal) conjugate was added in the second step. Following another incubation and wash, pre-trigger and trigger solutions are then added to the reaction mixture. The resulting chemi-luminescent reaction was measured as relative light units (RLUs). A direct relationship exists between the amount of troponin-I in the sample and the RLUs detected by the Architect i System optics. The concentration of troponin-I is read relative to a standard curve established with calibrators of known troponin-I concentrations.• Galectin-3 levels determined using commercially available enzyme-linked immune-sorbent assay (human galectin-3 ELISA) kit (WKEA MED supplies corp. USA) according to manufacturer’s protocol [provided by WKEA MED supplies corp. New York. USA] and were measured on thermo scientific multiskan EX microplate photomter reader. Normal value: 0.7-22ng/ml. Calibration of the assay was performed according to the manufacturer’s recommendations and values were normalized to a standard curve. This assay has high sensitivity (lower limit of detection 1.13ng/mL) and exhibits no cross-reactivity with collagens or other members of the galectin family. Commonly used HF medication; like angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, spironolactone, furosemide, acetyl-salicylic acid, warfarin, coumarines and digoxin have no interference with the assay[17].• Statistical analyses: Analysis of data was done using SPSS (statistical program for social science version 20 as follows: Ø Description of quantitative variables as mean ± SD and range Ø Description of qualitative variables as number and percentage Ø Unpaired t-test was used to compare quantitative variables, in parametric data (SD<50% mean)Ø One way ANOVA test was used to compare more than two groups as regard quantitative variables by comparison of means across quartiles of galectin-3 Ø Chi-square test was used to compare two groups as regard qualitative variablesØ Pearson Correlation was used to rank variables in correlation with Gal-3 and ctn I. Ø Receiver operator characteristic curve (ROC) analysis was performed. (Clinton et al., 1992)18 to find out the best cut off value of Gal-3 and troponin I to validate parameters as follow:Ø Sensitivity = true positive / (true positive + false negative) = ability of the test to detect positive cases.Ø Specificity = true - negative / (true negative + false positive) = ability of the test to exclude negative cases.Ø Positive predictive value (PPV) = true positive / (true positive +false positive) = % of true positive cases to all positive.Ø Negative predictive value (NPV) = true negative /true negative + false negative = % of the true negative to all negative casesØ P value >0.05 insignificant, P<0.05 significant and P<0.01 highly significant
4. Results
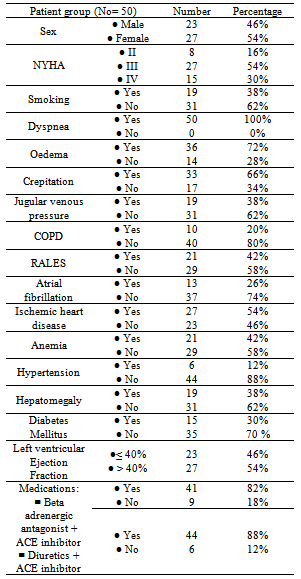
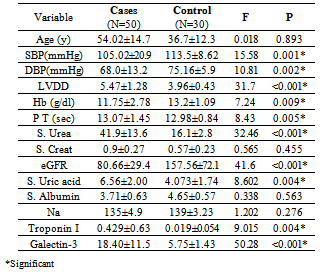
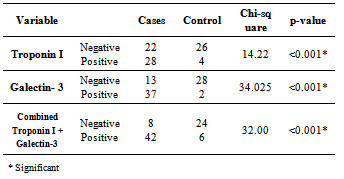
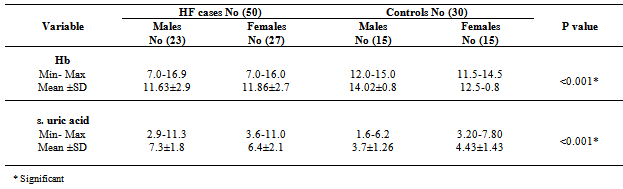
- The demographic characteristics and clinical data of all HF cases (table 1); the mean age 54.02±14.7 years, 27(54%) females and 23(64%) males. 8(16%) of HF patients was NYHA-class II and 42(84%) were class III and IV. 23(46%) of patients had LVEF ≤ 40%. All cases have dyspnea 50(100%), 19(38%) were Smokers, 36 (72%) had edema, 33(66%) had crepitations, 19(38%) with raised JVP and 19(38%) had hepatomegaly. Hypertension presents in 6(12%) of cases while anemia presents in 21(42%) of patients, 15(30%) were diabetics & 27(54%) had IHD, 10(20%) had COPD, 13(26%) had AF. All patients were on standard medication for HF, including beta blockers combined with ACEI were taken by 41(82%) of patients were as diuretics combined with ACEI were taken by 44(88%) of patients. A statistically significant difference between cases and controls; in relation to values of SBP, DBP, LVDD, Hb, Prothrombin Time, urea, eGFR, uric acid, Troponin I and galectin-3[table 2].
|
|
|
|
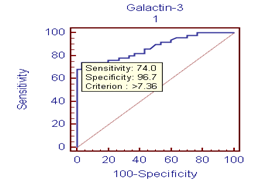 | Figure 1. ROC curve |
|
|
|
5. Discussion
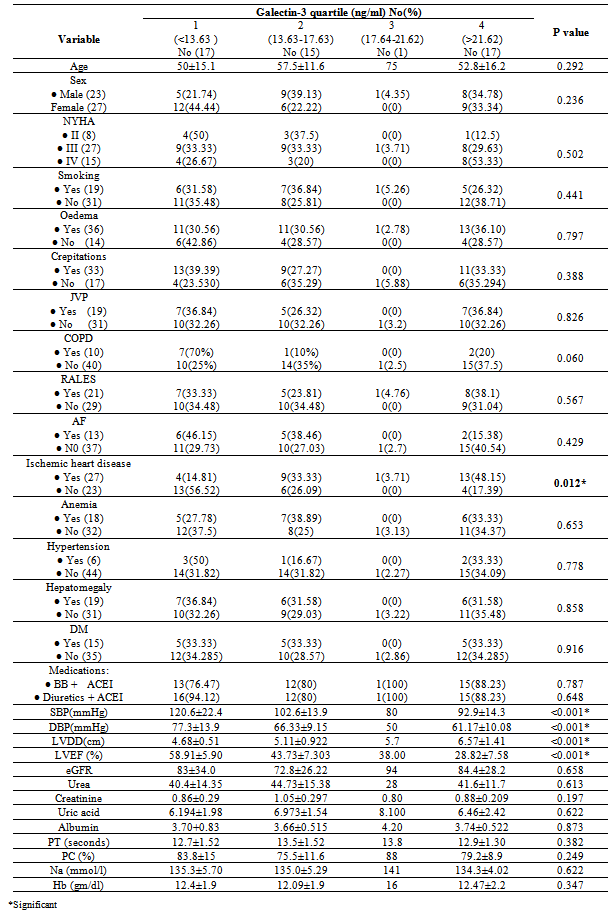
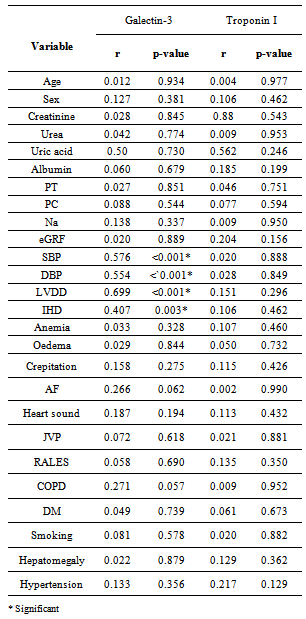
- It is clear that management of HF patients is a huge burden therefore, it is imperative to appropriately risk-stratify patients to identify those who need the closest follow-up. Traditional risk factors such as age, diabetes, and smoking, as well as symptom severity, can all indicate those at risk. However, these alone are often inadequate to stratify risk. As a result, novel blood-based biomarkers e.g. Gal-3 is directly involved in the patho-physiology of cardiac injury and progression to HF(19,20). Moreover, gal-3 appears to have direct clinical relevance in aiding diagnosis, risk stratification and prognosticate patients with HF, monitoring therapy, therapeutic modification and treating to targets in order to improve clinical outcomes[21].In our study; gal-3 and cTn I plasma levels were statistically significant higher in subjects with HF compared to control with mean gal-3 (18.40 ±11.5 ng/ml) among HF patients and (5.75 ±1.427 ng/ml) among control group (p value <0.001). And mean cTn I (0.429± 0.630 ng/ml) among HF patients and (0.019±0.0544 ng/ml) among control group (p value 0.004). This finding was in accordance with[11, 22-25].In our study; eGFR was statistically significant lower in subjects with HF compared to control (80.66± 29.4) vs (157.56±72.1) mL/min/1.73m2 (P < 0.001). This is in accordance with[20]. This finding is interesting since increased gal-3 is also associated with renal fibrosis (26) and the same process may thus affect both heart and kidneys. Renal dysfunction is one of the most powerful predictors of prognosis in HF and plays an important role in its pathophysiology and the ‘‘overlap’’ may thus also be mediated by gal-3[27].In our study, the baseline characteristics of all patients, according to quartiles of gal-3 levels showed highly statistically significant increase in gal-3 levels with the decreased LVEF, systolic and diastolic blood pressure (p value <0.001). There was statistically significant increase in gal-3 levels with the increased left ventricular end diastolic diameter "LVEDD" (p value <0.001). The increase in gal-3 levels had no statistically significant relation to age, gender, NYHA functional class, DM, COPD, smoking or use of medications (ACEI, BB &diuretics).In accordance with our study[8, 20, 28] showed that higher gal-3 levels were associated with other measures of HF severity, including lower systolic blood pressure. Another study[29] showed that patients with LVEDD progression had significant higher levels of gal-3 compared to patients showing regression of LVEDD with no significant correlation with age, sex, duration of HF or use of anti-failure medications. De Boer et al[30] showed that no-significant relation of use of anti-failure medications to plasma gal-3 quartiles. In contrast;[31] reported that gal-3 was not correlated with LVEF. In our study; there were a statistically significant difference between the studied groups as regard SBP, DBP, LVDD, urea (41.9±13.6) vs (16.1±2.8); (P=0.004), uric acid (6.56±2.0) vs (4.07±1.74); (P < 0.001), Hb (11.75±2.18) vs (13.2±1.09); (P= 0.009), Prothrombin time (13.07±1.45) vs (12.98±0.83) (P= 0.005).In our study; the baseline characteristics of all patients, according to quartiles of gal-3 show that the increase in gal-3 levels had no statistically significant relation to age, gender, NYHA functional class, DM, COPD, smoking or use of medications (ACEI, BB and diuretics). In accordance with our study;[8, 22, 31] but Lok et al[20] showed that gal-3 was correlated with age but no significant correlation with gender, NYHA, functional class, DM, COPD and smoking. On the other hands; another study by Lok et al[29] showed no significant correlation with age, sex, duration of HF or use of anti-failure medications. Felker et al[28] reported a higher gal-3 level that was associated with other measures of HF severity; including lower systolic blood pressure. In accordance with our study; de Boer et al[30] showed no-significant relation of use of anti-failure medications to gal-3 quartiles in contrast; he reported an increase in quartiles of gal-3 levels has statistically significant relation to age, eGFR, Hb and NYHA functional class.In our study; statistically high significant increase in gal-3 levels with the decreased left ventricular ejection fraction (LVEF), systolic and diastolic blood pressure (p value <0.001), and with the increase of both left ventricular end systolic diameter "LVESD" (p value 0.003) & left ventricular end diastolic diameter "LVEDD" (p value <0.001). In accordance with our study; Lok et al[29] showed that patients with LVEDD progression had significant higher levels of gal-3 compared to patients showing regression of LVEDD. In contrast (30, 31) showed that gal-3 was not correlated with LVEF. In our study; receiver-operating characteristic (ROC) analysis for gal-3 shows that the best cut off point which discriminates between disease and normal is gal-3 ≥7.36 with sensitivity 74% and specificity 96.7% and area under the curve (AUC) was 0.876 (p<0.0001) and positive predictive value (PPV) was 94.87% and negative predictive value (NPV) was 68.29%. And ROC analysis for ctn I positivity shows 56% sensitivity & 86.67% specificity and PPV 87.5% and NPV 54.17%. Whereas combined[ctn I positivity + gal-3 at (≥ 7.36 ng/ml)] shows 84% sensitivity & 80 % specificity and PPV 87.5% and NPV 75% i.e. improve gal-3 sensitivity and the negative predicator value so both together are valuable markers in predicting the HF severity.In accordance with our study; A study by van Kimmenade et al[22] showed that the ROC analysis for gal-3 for the diagnosis of HF showed AUC of 0.72 (p < 0.0001); with an optimal cutoff of 6.88 ng/ml yielding a sensitivity of 80% and a specificity of 52%. Another study by de Boer et al[32] showed AUC was 0.72 (P<0.0001); the optimal cut-off of gal-3 was 6.88 ng/mL which resulted in a reasonable sensitivity of 80% but a poor specificity of 52%.We aimed to correlate the results with some morbidity cofactors in HF patients such as anemia, hyperuricemia and hypernatremia. our study show statistically significant low Hb levels among HF patients compared to controls with mean Hb level among males (11.62±2.93 vs 14.02±0.84) (P<0.0001) & among females (11.86±2.68 vs 12.52±0.82) (P<0.0001); with the overall incidence of anemia was 21(42%) among HF patients, 52% of them was normochromic normocytic anemia and 48% of them was hypo-chromic microcytic anemia.In accordance with our study; a study by Groenveld et al[33] showed that 37.2% of patients with CHF were anemic concluding that anemia is associated with an increased risk of mortality in both systolic and diastolic CHF& should, therefore, considered as a useful prognosticator and therapeutic strategies aimed to increase hemoglobin levels in CHF . So these patients should be investigated to improve morbidity and potentially mortality in them. Another study by Tehrani et al[34] showed that anemia was present in 55% of the CHF patients.In our study serum uric acid levels show statistically significant high levels among HF patients with mean uric acid level among males (7.24±1.76) and among females (6.43±2.04) compared to controls (3.71±1.26 among males) and (4.43±.1.43) among females. In accordance with our study; Ogino et al[35] showed that serum uric acid was significantly increased (p<0.01) in relation to NYHA HF severity (control 5.45± 0.70 mg/dl, NYHA I 6.48± 1.70 mg/dl, NYHA II 7.34±1.94 mg/dl, NYHA III 7.61±2.11 mg/dl, p<0.01) indicating that hyperuricemia was common in CHF, and it is a strong independent marker of prognosis. This may be explained by the fact that serum uric acid level is an index of oxidative stress in the human body (36), and was known to contribute to endothelial dysfunction by impairing nitric oxide production [37]. Serum uric acid has shown to be inversely correlated with the measures of functional capacity and maximal oxygen intake in heart failure patients[38]. Increased oxidative stress results from an imbalance between reactive oxygen species and endogenous antioxidant defense mechanisms. The imbalance can exert profoundly deleterious effects on endothelial function as well as on the pathogenesis and progression of HF[39]. Among patients with chronic HF, serum uric acid concentrations were associated with greater activity of superoxide dismutase and endothelium dependent vasodilatation[40]. In our study serum sodium showed non-significant elevations among HF patients as all patients were on diuretic therapy.The main limitation of the current study was its relatively small size. Furthermore, we measured gal-3 on a single time point and thus can only speculate on its importance over time. Limitations of our study include the fact that gal-3 did not correlate with severity of dyspnea as categorized by the NYHA functional classification. Such a lack of correlation makes, according to some investigators, a new diagnostic serum marker less suitable for clinical practice[41]. However, a prognostic marker in HF actually does not necessarily correlate with NYHA functional classification, which was according to some investigators, rather subjective[42] and merely a “crude estimation of a patient’s functional capacity”[43]. Because of these limitations, we regard our study mainly as a hypothesis-generating study.
6. Conclusions
- Gal-3 is a novel biomarker when used in HF patients help improving prognostication and its combined assessment with ctn I help to tailor the most appropriate treatment strategy on a more individualized basis and using gal-3 as therapeutic target in treatment of chronic HF in future is suggested. In additions; gal-3 assessment with ctn I may allow the identification and treatment of patients with suspected HF e.g. coronary artery disease who display adverse remodeling and, in doing so, prevent the development of HF.
Abbreviations
- ACE: Angiotensin-converting enzymeAF: Atrial fibrillationAlb: AlbuminBB: Beta-blockerCAD: Coronary artery diseaseCBC: Complete blood countCHF: Congestive heart failureChemiluminescent microparticles immunoassayCOPD: Chronic obstructive pulmonary diseaseCtn: Cardiac troponinsDBP: Diastolic blood pressureDM: Diabetes mellituseGFR: estimated Glomerular filtration rateGal-3: Galectin-3IHD: Ischemic heart diseaseLVEF: Left ventricular ejection fractionMI: Myocardial infarctionNPV: Negative predictive valueNYHA: New York Heart AssociationPPV: Positive predictive valueROC curve: Receiver-operating characteristic curveSBP: Systolic blood pressure
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML