-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2013; 3(4): 82-87
doi:10.5923/j.cmd.20130304.02
A Business Impact Study of the Use of a Supersaturated Calcium Phosphate Oral Rinse (SCPOR) in the Prevention and Treatment of Oral Mucositis
D. Wayne Taylor
The Cameron Institute and McMaster University, Hamilton, L7L 5R8, Canada
Correspondence to: D. Wayne Taylor, The Cameron Institute and McMaster University, Hamilton, L7L 5R8, Canada.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Oral mucositis (OM) is an inflammation of mucous membranes in the mouth with symptoms ranging from redness to severe ulcerations and pain. It is a condition that affects as many as 45,000 Canadian cancer patients annually, and around 70% of patients undergoing conditioning therapy for bone marrow transplantation (BMT). Almost all patients receiving radiation therapy to the head and neck areas develop OM. Basic oral care is often not enough to reduce the duration or severity of OM in cancer patients. The author conducted a business impact study for Canadian hospitals and cancer centres of the use of a prescription, supersaturated, calcium phosphate, oral rinse (SCPOR) in the prevention and treatment of oral mucositis that occurs due to high-dose chemotherapy in bone marrow transplant patients as well as in head and neck cancer patients receiving radiation therapy. Treatment of OM with the SCPOR for BMT patients not only provided positive, clinical results but net savings of $1,585 for a return on investment of 238.3%. The minimal net savings per head and neck cancer patient, a patient who would also be receiving clinically better care for OM, would be $663 for a return on investment of 49.8%.
Keywords: Oral Mucositis, Oral Rinse, Calcium Phosphate, Electrolytes, Business Impact
Cite this paper: D. Wayne Taylor, A Business Impact Study of the Use of a Supersaturated Calcium Phosphate Oral Rinse (SCPOR) in the Prevention and Treatment of Oral Mucositis, Clinical Medicine and Diagnostics, Vol. 3 No. 4, 2013, pp. 82-87. doi: 10.5923/j.cmd.20130304.02.
Article Outline
1. Introduction
- Oral mucositis (OM) is an inflammation of mucous membranes in the mouth with symptoms ranging from redness to sever ulcerations. Extrapolating from American data, it is a condition that affects as many as 45,000 Canadian cancer patients each year[1], and around 70% of patients undergoing conditioning therapy for bone marrow transplantation (BMT)[2]. Approximately 40% of patients receiving chemotherapy develop some form of OM during the course of their treatment[1]. Almost all patients receiving radiation therapy to the head and neck areas develop OM[3].The incidence of severe OM (grades 3/4) often exceeds 50% of patients receiving radiation to the head and neck, and 60% of BMT patients whose conditioning therapy includes total body irradiation[4]. Severe OM rates as high as 98% have been observed in patients that have received chemotherapy combined with radiation to treat head and neck cancer[5]. OM is one of the major causes of severe pain and debilitating toxicity associated with high-dose chemotherapy and radiation therapy[6]. OM can also lead to dysphagia, infection(s), and depression – all of which are co-morbidities that can inhibit the progress of cancer treatment. Nurses have identified OM as the most debilitating problem associated with cancer treatment[7].Since many treatment regimens include both markedly and mildly mucotoxic agents, predicting the potential for mucositis to develop can be difficult[2].Mucositis is usually an acute event that lasts typically 16 days, in Bone Marrow Transplant (BMT) and solid tumour patients, after the start of cytotoxic treatment with healing commencing about day 12[8]. Timing is different for OM resulting from radiation therapy as radiation therapy typically is administered over an extended period of time[3]. No matter the type of tumour or treatment. OM can have rate-limiting effects on treatment regimens, as discussed below.There is an advanced aqueous electrolyte solution in therapeutic use in the United States and Europe that is clinically proven[6] to be a significant adjunct in the management of mucositis associated with radiation therapy and high-dose chemotherapy. This solution is a prescription, supersaturated, calcium phosphate, oral rinse (SCPOR) and provides a proven alternative for patients and healthcare professionals who are unsatisfied with current options that focus on good oral hygiene, antibiotics when appropriate, and the management of oral pain[9]. The author conducted a business impact study for Canadian hospitals and cancer centres of the use of SCPOR in the prevention and treatment of oral mucositis that occurs due to high-dose chemotherapy in bone marrow transplant patients as well as oral mucositis in head and neck cancer patients receiving radiation therapy.
2. Clinical and Economic Implications of OM in the Literature
- According to 42% of patients on high-dose chemotherapy and 38% of patients treated with head and neck irradiation, oral mucositis is the most troubling side effect of their therapy[10]. Severe OM can complicate the management of cancer, interrupt cancer treatments, and compromise outcomes[5]. OM may lead to missed doses, reduced doses, or treatment failure[2]. OM has also been correlated with: increased risks for life-threatening systemic infections in these immunocompromised patients[11]; a predisposition of patients to potentially fatal septicemia[3]; the use of opioid analgesics for pain relief along with associated co-morbidities[3]; and the requirement for total parenteral nutrition (TPN)[5], all resulting in increased length of hospital stay (LOS) and associated costs[12].Healthcare economic studies usually look at three types of costs: direct costs, both medical and non-medical; indirect costs such as lost wages due to illness; and, intangible costs which may include psychosocial and societal costs. A business impact study, such as this one, is only concerned with direct costs.Evaluations of the economic costs of particular treatment side-effects, as in the case of OM, are rare; cost-effectiveness studies are virtually non-existent. There have been only a few studies examining the costs of treating oral mucositis in head and neck cancer or bone marrow transplants.Incremental costs due to OM result from additional treatment costs that may include mucositis pain relief, IV hydration for mucositis-related dehydration, parenteral nutrition, infection treatment, increased professional time, longer hospital stay, and so on.In a 1996 U.S. study, the mean incremental cost of OM in head and neck cancer ranged from a low of $2,949 to a high $4,037 per treatment episode[13]. Assuming a 5% healthcare inflation multiplier[14], these costs would be $5,839 and $7,993 respectively in 2010 dollars. The range in mean incremental costs was a result of the two costing methodologies used based largely on, alternatively, U.S. Medicare reimbursement figures and billed charges, representing hospitalization costs – the single largest contributor to incremental costs followed by drug costs, support costs and MD/RN time. The difference between Medicare reimbursement fees and billed charges was significant at the hospital studied given that billed charges in the U.S. generally must also include allowances for bad debt, uncompensated care, and capital costs which Medicare does not reimburse.In both the low and high cost halves of the above study, patients with severe OM had statistically significant higher costs for drugs and support (pain medication, G-tubes, IV hydration, nutritional supplements)[13].Given that Medicare reimbursements are more in line with Canadian reimbursement figures, rather than billed charges, the lower mean incremental cost should be seen as instructional in studying OM treatment costs in head and neck cancer in Canada. At the time of writing, the Canadian dollar was just about at par with the U.S, dollar so no exchange rate calculations have been made.Two more studies were conducted a decade later, again looking at the costs of treating OM with head and neck cancer. In both studies over two-thirds of the patients studied had severe, grad 3/4 OM. The 2007 study identified the incremental costs, in 2006 dollars, of treating OM as being $1,700 for grade 1/2 OM and $6,000 for grade 3/4 OM[15]. In 2010 dollars those amounts would be $2,066 and $7,293 respectively. The 2008 study calculated incremental costs, in 2005 dollars, for treating only severe OM for head and neck cancer as $17,244[16]. In 2010 dollars that amount would be $22,008.Severe OM is also associated with significantly worse clinical and economic outcomes, including mortality, in BMT[12]. From the patient’s viewpoint, OM is often the single most debilitating side-effect of a transplant. A U.S. study concluded that, in BMT patients, OM was statistically correlated with an increase in the: incidence of significant infection; days on TPN; days receiving injectable narcotics; length of stay in hospital; total costs; and, rate of mortality within 100 days of the first day of conditioning[12]. The treatment of OM in hematopoietic stem cell transplantation (HSCT) cost, on average, a total of $42,749 in this 2001 study[12]; in 2010 dollars that would be $66,318.
3. Treatment of Oral Mucositis–Clinical and Economic Outcomes
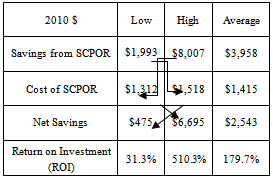
- Oral Mucositis is an underestimated and under-treated condition that severely affects quality-of-life and success of cancer treatment. Basic oral care may not be enough to treat OM in cancer patients[17]. The development of effective treatments to prevent and/or reduce the severity of OM has been slow. At present there is considerable variation amongst OM treatment guidelines. Few interventions have proven effective in reducing the duration or severity of OM.SCPOR is one agent that has been proven to reduce the frequency and severity of OM[6]. A unit dose product, SCPOR is not for systemic use but should be initiated at the onset of cancer treatment.In a prospective, randomized, double-blind,placebo-controlled trial of treating OM in hematopoietic stem cell transplantation (HSCT) patients, the SCPOR-treated patients, in conjunction with standard oral care, had significantly reduced incidence, intensity and duration of OM over those patients who had received a fluoride rinse and standard oral care[6]. Amongst the SCPOR-treated patients, 40% did not develop OM whereas only 19% of the control group avoided OM. This study also demonstrated that the treatment of OM by SCPOR statistically significantly reduced the: number of days of mucositis (3.72 days versus 7.20 days); duration of pain (2.86 days versus 7.67 days); number of days of morphine (1.26 days versus 4.02); and dose of morphine (34.54 mg versus 122.78)[6].SCPOR proved to be superior to placebo in reducing the frequency, intensity, and duration of OM in patients undergoing HSCT.SCPOR is an oral rinse not intended to be ingested. The Federal Drug Administration (FDA) approved SCPOR as a device indicated as an adjunct to standard oral care in treating the mucositis that may be caused by radiation or high-dose chemotherapy. This indication received premarketing clearance in 2003. There are no known contraindications. No adverse effects have been reported following the use of SCPOR. If accidentally swallowed, no adverse effects are anticipated. There are no known interactions with medicinal or other products[18].A retrospective study, covering a two-year time period, of two groups of non-Hodgkin’s lymphoma patients who had received autologous peripheral blood stem cell transplantations revealed that the group that received standard care and SCPOR for the treatment of their OM fared better than those who only received standard care[19]. The mean number of days on patient-controlled analgesia (PCA) was 3.8 for SCPOR patients and 5.5 for those receiving only standard care. The mean daily dose of PCA hydromorphone was 9.5 mg for SCPOR patients versus 12.5 for standard care patients. Average length of hospital stay was reduced 1.5 days (~10%) for SCPOR-treated BMT patients from 16.2 days to 14.6 days for a cost savings of $6,141 in 2010 dollars. The study concluded that SCPOR had a positive effect on both clinical and economic outcomes of OM for BMT patients.From February 2007 to May 2008, a retrospective, match controlled study of SCPOR versus supportive care for OM in head and neck cancer patients was conducted to determine the effectiveness of SCPOR for reducing the incidence and severity of OM – which it did[14] (see Figure 1). Only 38% of patients treated with SCPOR developed severe OM whereas 71% of the control patients developed severe OM. Conversely, 62% of SCPOR-treated patients contracted low grade OM versus only 29% of the control patients.In addition, no adverse effects associated with the use of SCPOR were observed.The study also concluded that the use of SCPOR reduced the costs associated with treating OM through reduced hospitalization stay and reduced associated supportive care costs such as fewer PEG tube placements. Using the two different cost models and the associated cost data from the two articles cited above[15],[16] the study by Miyamoto et.al.[14] calculated the cost savings from treating OM with SCPOR as being between $1,722 (32.5% of the costs of the control patients) and $6,917 (46.5%). In 2010 dollars those savings would be $1,993 - $8,007. The weighted average savings per SCPOR-treated patient was 37.1% of the control patient cost, or $3,958 in 2010 dollars.The cost of treatment with SCPOR is not prohibitive. In 2008 the cost of SCPOR was US$154.00/30 doses or US$559.36/120 doses. According to another 2007/2008 study, head and neck cancer patients treated for OM with SCPOR were administered, on average, 4.56 rinses per day and the average course of treatment was for 8 weeks[20]. Both the incidence and severity of OM was significantly less with the use of SCPOR. High levels of compliance were observed due to the ease of administration, as well as high levels of both patient and physician satisfaction. In this study, the average cost of treating OM with SCPOR in head and neck cancer patients was $1,190 - $1,311 depending upon the dose size purchased. In 2010 dollars those costs would be $1,378 - $1,518 (see Table 1).
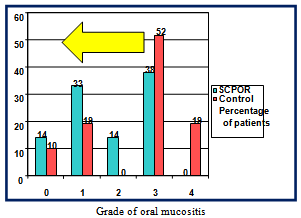 | Figure 1. Results of SCPOR vs. control (supportive care) |
|
4. Business Impact of SCPOR for Canadians
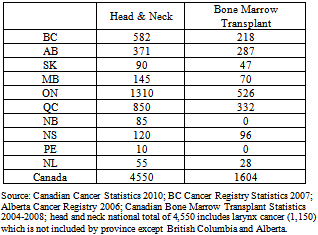
- In 2005 there were 3,756 reported cases of head and neck cancer in Canada[22]. By 2010 it had been estimated that figure would grow to 4,550 cases[23], exhibiting an annual growth rate of 4% versus a population growth rate of 1.5% per annum[24]. Between 2004 and 2008 bone marrow transplants numbered as many as 1,600 in one year[25] .
4.1. Treatment of OM with SCPOR in Bone Marrow Transplant Patients
- Assuming there are 1,600 BMT patients per year in Canada, and that the most significant proxy for better clinical and economic outcomes from treating OM in these patients with SCPOR is the 10% (1.5 days) reduction in length of hospital stay (LOS), then the direct cost saving would total 2,400 patient bed-days.The “fully loaded” average cost of a BMT bed-day ranges from $2,500 to $4,500[26]. Again, assuming a 10% reduction in LOS, treating OM with SCPOR for bone marrow transplants would save a hospital $3,750 - $6,750 per patient, depending upon their cost structure, and provide better clinical outcomes.Given the distribution in Table 2 of BMT procedures across the country, a weighted national average, fully loaded cost of a BMT bed-day would be approximately $3,677. At a reduced LOS of 1.5 days that would provide an overall savings of $8,824,800 - with improved clinical outcomes.The Canadian pricing of SCPOR was $665 per month of therapy. Treatment of OM with SCPOR in the case of BMT patients normally lasts 4 weeks; therefore it is safe to say that the cost of treatment per patient would be $665.
|
4.2. Treatment of OM with SCPOR in Head and Neck Cancer Patients
- Given the variability in the American cost savings data in using SCPOR for head and neck cancer this portion of the study assumed the most conservative savings of $1,993. In the case of head and neck cancer patients, a two month course of treatment is usually indicated so the cost of the SCPOR per patient would be $1,330. The minimal net savings per head and neck cancer patient (savings minus cost of SCPOR), a patient who would also be receiving clinically better care for OM, would be $663 for an ROI on SCPOR of 49.8%.Given 4,550 cases of head and neck cancer, national net savings would amount to a minimum of $3,016,650.
5. Conclusions
- Using SCPOR to treat OM would yield combined national savings for both BMT and head and neck cancer of $10,801,450, while providing better clinical care for cancer patients suffering from OM.Unfortunately, adequate data was not available for head and neck cancer for a case example to be constructed. There was plenty of data available for the top four cancers (breast, colorectal, lung and prostate) but very little for the others – either known or being collected. Likewise the costs of treating cancers other than the top four were not known at the time of this study. Future research in this area is required for a better understanding of the costs and benefits from innovative treatment options.
ACKNOWLEDGEMENTS
- The Industrial Research Assistance Programme of the National Research Council of Canada is acknowledged for its financial support of this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML