-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2013; 3(2): 11-17
doi:10.5923/j.cmd.20130302.01
The Many Faces of Lupus: An Approach to the Assessment of a Lupus Patient
Melba I. Ovalle , M. D.
Nova Southeastern University, Physician Assistant Program- Orlando Campus, USA
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
One of the first steps in evaluating a patient with lupus is to recognize that there are various subtypes of lupus. Determining which variant of lupus in afflicting the patient will determine the extent of disease involvement and even prognosis for the patient. Certain anti-nuclear antibodies and biomarkers for lupus have been shown to correlate with disease activity. Recognizing the prognostic indicators of active lupus can help guide a clinician in choosing the appropriate pharmacologic treatments for their lupus patients. This clinical review and summary of the literature was derived from medical and online databases such as PubMed , Cochrane review, up-to-date, the American College of Rheumatology and American College of Physicians Visual Diagnostic Logical Imaging resources. The following is an evidence-based, objective and balanced summation of this literature review along with the author’s own rheumatologic clinical experience over the past 25 years. For the first time in decades, consensus guidelines have been established for the management of lupus nephritis. In addition, the first Food and Drug Administration-approved biologic agent, belimumab, is now available for the treatment of certain manifestations of systemic lupus erythematosus. The reader will be able to recognize at the bedside the various subtypes of lupus, parameters of disease activity and the appropriate selection of treatment modalities.
Keywords: Antiphospholipid, Autoantibodies, Lupus
Cite this paper: Melba I. Ovalle , M. D. , The Many Faces of Lupus: An Approach to the Assessment of a Lupus Patient, Clinical Medicine and Diagnostics, Vol. 3 No. 2, 2013, pp. 11-17. doi: 10.5923/j.cmd.20130302.01.
Article Outline
1. Introduction
- The word lupus means “wolf” in Latin. Historically, physicians often thought the facial lesions of lupus patients resembled the bites of a wolf. The images of this disease often raised fear and frustration among patients as well as their doctors. Over the past century, the recognition and classification of various subtypes of lupus, the fractionation of auto-antibodies and their prognostic significance have facilitated the recognition and management of the various subsets of lupus. For the first time in several decades, a biologic agent approved by the U.S. Food and Drug Administration has been added to the treatment armamentarium for systemic lupus erythematosus (1).
2. First Steps in the Evaluation of a Lupus Patient
- One of the first steps in the evaluation of a patient is to recognize which population of patients is likely to succumb to a disease. Lupus is a prototypical autoimmune disease which has a female preponderance. Peak incidences occur during one’s most productive years of life: 15 through 45 years of age (2). As with many diseases, Hispanics and African-Americans are afflicted more often than Caucasians and with more severe disease (3). The pathogenesis of this disease has been recognized as probably being multi-factorial, with a genetic predisposition triggered by hormonal and environmental factors. The Major Histocompatibility Complex (MHC) Human Leucocyte Antigen (HLA) DR 2, DR 3 and B8 allelles serve as antigenic determinants, known as epitopes, contributing to the formation of pathogenic auto-antibodies due to dysregulation of self-tolerance, apoptosis and cellular inflammation (4).The next step is to be alert for the environmental triggers that generally pre-date and/or accompany the disease presentation by performing a thorough review of systems. Antecedent infections, stress, hormonal therapies, sunlight and certain medications have been recognized as inciting triggers of lupus (5). You will learn in this monograph how certain triggers will lead to a specific type of lupus presentation, e.g. exposure to procainamide can lead to a drug-induced lupus (6).
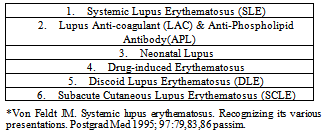
3. Subtypes or Variants of Lupus
- There are numerous subtypes of lupus as shown in Table 1 (7).
|
3.1. Systemic Lupus Erythematosus (SLE)
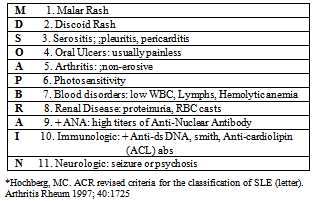
- The most severe and widespread form of lupus is the systemic form, which generally involves multiple organs such as the kidney, lungs, heart, brain and hematopoietic system as well as the skin and joints. The female: male ratio is 8:1, with a predisposition in minority groups such as African-Americans and Hispanics (8). The bi-modal peak incidence of 15 and 45 yrs of age is possibly due to the variation in hormones at that time (9). As mentioned earlier, genetic predisposition does exist with a 24-30% concordance among monozygotic twins, 2-5% concordance among dizygotic twins suggesting other factors contributing to SLE other than genetics (9). The pathogenesis of SLE is highly complex. Predominantly, there is an up-regulation of CD4 helper cell activity with sub-optimal CD8 suppressor cell function, over-expression of B cells with proliferation of auto-antibodies, which in turn, form immune complexes depositing on tissues causing cellular injury (10). SLE has a myriad of presentations ranging from mild, localized disease to severe multi-organ involvement abruptly orsequentially over the course of months to even years. This poses a challenge to practitioners as SLE can be a great mimicker of many diseases. The 1997 American College ofRheumatology revised criteria for the classification of Systemic Lupus Erythematosus offers a list of the most typical clinical features found in this systemic form (Table 2) (11).
|
|
|
3.2. Lupus Anti-coagulant and the Anti-Phospholipid Antibody Syndrome (LAC or APL)
- Contrary to its name, the lupus anticoagulant (LAC) is actually an immunoglobulin with thrombotic properties binding to the phospholipids of cell membranes as well as plasma cell proteins such as beta 2 glycoprotein I. It is an inhibitor globulin which causes an increase in activated partial thromboplastin time when testing a patient’s serum with LAC. This and other anti-phospholipid (APL) antibodies cause a hypercoagulable state resulting in frequent miscarriages, arterial and venous thrombosis and thrombocytopenia (22). APL antibody can exist by itself and often these patients will have a history of recurrent spontaneous abortions as the sole complaint (23). A typical rash, livedo reticularis, is characteristic in these patients (Figure 1).
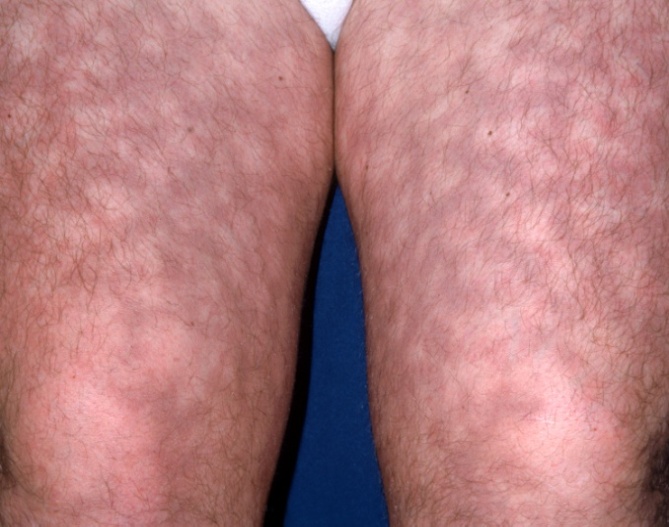 | Figure 1. Livedo Reticularis |
3.3. Neonatal Lupus
- Neonatal lupus results from the vertical transmission of the mother’s auto-antibodies to the fetus. The SSA/Ro, sometimes SSB/La, IGG antibodies travel across the placenta and disseminate throughout fetal tissue. Serious manifestations can occur when these auto-antibodies bind to fetal cardiac tissue and cause congenital heart block with third degree heart block, bradycardia and/or myocarditis. Thrombocytopenia and leukopenia may also occur. The rash is the least worrisome clinical manifestation, which often resolves as the maternal antibodies wane from the infant in approximately 6 to 8 months (Figure 2). Treatment applies to symptoms present (25).
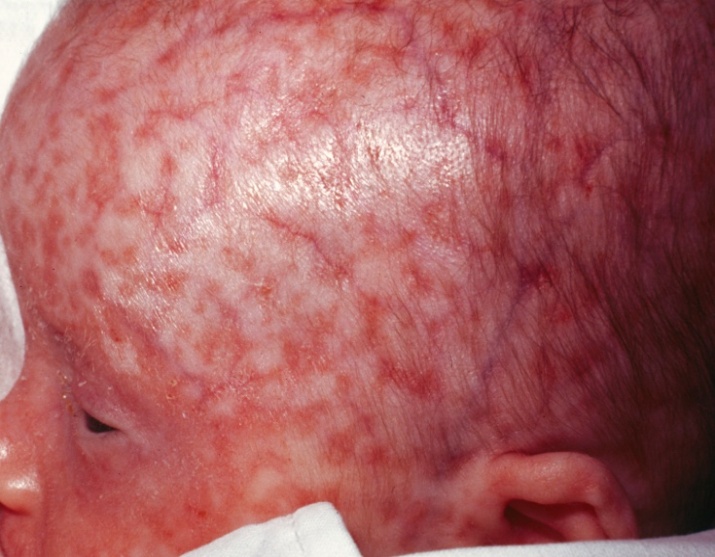 | Figure 2. Neonatal Lupus |
3.4. Drug-induced Lupus Erythematosus
- Many patients have reactions to various medications and can even present with auto-immune complications. The pathogenesis of drug-induced lupus erythematosus is not well understood. However, a common theory is that certain drugs act as haptens due to their oxidative metabolites, trigger the formation of auto-antibodies against histone proteins and cause clinical manifestations from mild, localized disease (serositis, pleuritis) to a diffuse, systemic form of lupus (26). Although there are numerous medications causing the formation of anti-histone antibodies and thus, drug- induced lupus, definitive drug causes have been identified as procainamide, minocin, hydralazine and biologics such as etanercept (27). Treatment is focused on removal of the drug and supportive care until symptoms resolve.Steroids are rarely necessary.
3.5. Discoid Lupus Erythematosus (DLE)
- Mucocutaneous forms of lupus cause significant cosmetic difficulties for patients. Most of these cutaneous forms are localized to the face, cheeks, upper arms and chest. There is tremendous variability of the rashes, ranging from the classic butterfly rash seen in SLE to fixed lesions which can scar and disfigure if not treated promptly as in Discoid Lupus Erythematosus (Figure 3).DLE is mostly localized to the skin and rarely generalizes or evolves to a systemic form of lupus. Diagnosis is confirmed by a skin biopsy revealing hyperkeratosis, follicular plugging and immune mononuclear cells at the dermal-epidermal junction (28). The Anti-nuclear antibody (ANA) so characteristically sensitive in the detection of lupus tends to be negative in this variant. Sunscreen protection, intra-lesional steroid injections and/or anti-malarials are the usual modalities of treatment for DLE.
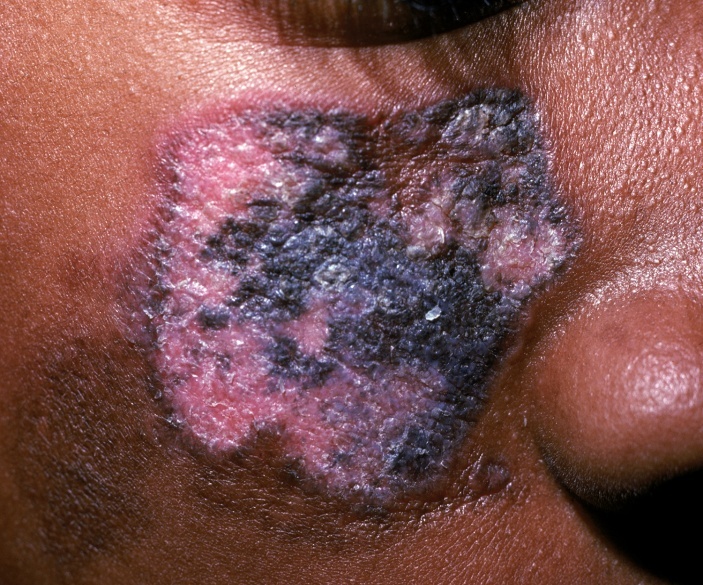 | Figure 3. Discoid Lupus Erythematosus |
3.6. Subacute Cutaneous Lupus Erythematosus (SCLE)
- Subacute Cutaneous Lupus Erythematosus (SCLE) is another form of mucocutaneous lupus. This cutaneous form of lupus is more common in Caucasian females. The papulosquamous rash tends to be annular, circular and non-scarring (Figure 4).
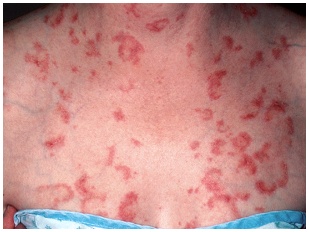 | Figure 4. Subacute Cutaneous Lupus Erythematosus |
4. Monitoring Parameters for the Management of Lupus
- As in most chronic diseases, a thorough history and physical examination are paramount for the management of lupus. Educating the patients about the early signs of a lupus flare can also allow for acute intervention and prevention of more serious disease. Flare-ups of lupus can present as worsening fatigue, low-grade fevers, arthralgias, myalgias and other constitutional symptoms. The butterfly rash may appear more intense and/or other rashes may become more disseminated. An antecedent infection, recent drug use or a photosensitivity reaction can often trigger these flares (5). This should prompt the practitioner to obtain a panel of acute phase reactants and biomarkers. The acute phase reactants such as sedimentation rate and C-reactive protein usually correlate with disease activity. There have been some studies suggesting the high sensitivity C-reactive protein (hs-CRP) has been used as a risk assessment for cardiovascular disease in lupus patients (30). Other serologic biomarkers such as elevated anti-ds DNA antibodies and low complement levels such as CH50, C3 and C4 have been shown to correlate strongly with active lupus, particularly with renal disease (31). A complete blood count, metabolic panel and urinalysis looking for involvement of target organs should also be obtained in the evaluation of a lupus patient. Further imaging and diagnostic studies are reserved for those patients for more severe complaints or disease state.
5. Pharmacotherapy and the Indications for Treatment of lupus
- The types of therapy that will be instituted for a lupus patient will depend on the degree of clinical presentation and severity of disease. Patients that have arthralgias and myalgias may be managed by non-steroidalanti-inflammatory agents. Because lupus patients have a propensity for drug-induced reactions, judicious use of medications and weighing of the risks and benefits of such medication is paramount. It is worth noting that cases of meningoencephalitis due to the use of ibuprofen in patients with autoimmune diseases, especially lupus, have been reported (32). Because corticosteroids have an abrupt onset of action, their role is reserved primarily for patients requiring immediate therapy and/or control of their disease state. It is often employed as bridging therapy with a subsequent taper once disease modifying drugs have become effective in these patients. The actual mechanisms of action of corticosteroids are complex and go beyond the scope of this monograph. Suffice to note that the general consensus is that corticosteroids’ mechanism of action and side effects are due to the modulation of gene transcription (33). It is never appropriate for an abrupt termination of steroids, even with those patients on low steroid doses, as this may lead to an addisonian crisis due to the suppression of the adrenal glands. Long term use of steroids is discouraged due to their inherent side effects such as diabetes mellitus, osteoporosis, avascular necrosis, glaucoma and cushingoid features. Disease modifying anti-rheumatic drugs (DMARDs) are used for maintenance and long-term therapy of lupus patients. The profiles of these drugs range from slow-acting anti-rheumatic drugs (SAARDs) such as the anti-malarials (hydroxychloroquine), to anti-metabolites (methotrexate), to more immunosuppressive drugs (cyclophosphamide, mofetil mycophenalate). Lupus patients with rashes and non-erosive arthritis benefit from the use ofhydroxychloroquine. Hydroxychloroquine is now considered the gold standard for the control of this disease due to its beneficial effects on the suppression of flares, controllingdyslipidemias and prolongation of survival rates in lupus patients (34). Methotrexate is often used as a steroid-sparing agent and/or as a maintenance drug after induction therapy with cytotoxic agents (35). Lupus patients with more severe systemic states involving renal, neurologic and/or vasculitis are treated with cyclophosphamide, mofetil mycophenalate or azathioprine (36, 37). For the first time in over half a century, a FDA-approved biologic agent, belimumab (Benlysta), now available for SLE patients, is a human monoclonal antibody which functions as a B-lymphocyte stimulator (BLyS) - specific inhibitor, blocking the binding of soluble BLyS, a B-cell survival factor, to its receptors on B cells (1). It has long been speculated that B-cell modulation may be beneficial for lupus patients which has been the impetus for more investigational agents (38). Belimumab was studied and approved for adult patients with active, autoantibody-positive SLE receiving standard therapy such as NSAIDs, steroids, anti-malarials and immunosuppressives. It is administered as infusion therapy: IV 10 mg/kg at 2-week intervals for the first 3 doses and at 4-week intervals thereafter. The trial noted the adverse effects of increased infections, nausea, diarrhea, depression and even mortality more than placebo (39). One of the major criticisms of the BLISS study was the exclusion of lupus patients with kidney and CNS involvement from the trial. In addition, African American & Hispanic patients in the study were a small sample size and did not appear to respond to treatment with belimumab (Benlysta). The study lacked sufficient numbers to establish a definite conclusion in these particular patients. However, extension trials for this subpopulation of patients may help evaluate the safety and effectiveness of Benlysta.
6. Conclusions
- Lupus is a chronic inflammatory autoimmune disease which can affect any organ system, but mainly involves the skin, joints, kidneys and nervous system. The diagnosis of lupus and the recognition of its various subtypes must be based on the proper constellation of clinical findings and laboratory evidence. Recognition of the pathogenic auto-antibodies and their targeted organs can help predict outcomes and treatment responses. Continual monitoring parameters include periodic follow-up and laboratory testing to detect signs and symptoms of any new organ-system involvement. Overall treatment will depend on disease severity and organ involvement weighing the risks and benefits of each treatment modality.
ACKNOWLEDGEMENTS
- I would like to thank Dr. Augusto Montalvo, Assistant Clinical Professor of Dermatology, Feinberg School of Medicine, in Chicago, Illinois for his assistance in editing. I also thank LogicalImages.com for assistance in obtaining these photographs.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML