-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2013; 3(1): 1-5
doi:10.5923/j.cmd.20130301.01
Serum HER-2/neu Reference Intervals in a Multiethnic Malaysian Population
Alicezah MK 1, Fathimah M 1, M Thevarajah 1, M Naicker 1, Yip CH 2, Taib NA 2, Rozita AM 3
1Department of Pathology, Faculty of Medicine, University Malaya, Lembah Pantai, 50903 KL, Malaysia
2Department of Surgery, Faculty of Medicine, University Malaya, Lembah Pantai, 50903 KL, Malaysia
3Department of Oncology. Faculty of Medicine, University Malaya, Lembah Pantai, 50903 K L, Malaysia
Correspondence to: M Thevarajah , Department of Pathology, Faculty of Medicine, University Malaya, Lembah Pantai, 50903 KL, Malaysia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Amplification of HER-2/neu gene in breast cancer carries poor clinical prognosis, but its detection is important in managing the disease. Immunohistochemistry (IHC) is used traditionally to assess the HER-2/neu status, but this technique requires high quality tissue samples. Serum HER-2/neu provides an alternative non invasive option to the assessment of Her-2/neu status. To establish the reference range values of serum HER-2/neu concentration in multiethnic Malaysian women and to evaluate the association between serum HER-2/neu with the clinicopathological parameters. The study population comprised of 40 patients with breast cancer, 40 with benign breast disease (BBD) and 102 healthy volunteers. The serum- HER-2/neu concentration was measured and tissue samples from breast cancer patients were evaluated for HER-2/neu by immunohistochemistry (IHC). The mean (SD) for breast cancer, BBD, and healthy volunteers were 11.11(2.22), 8.73(2.57) and 7.88 (3.35) ng/ml, respectively in contrast to the manufacturer defined reference range of 15ng/ml. The The serum HER-2/neu concentration displayed no significant correlation with the clinicopathological parameters. No statistically significant association was noted between serum HER-2/neu concentration and tissue HER-/neu (p>0.05). Serum HER-2/neu cut off values though provided by the manufacturer was noted to be lower in our cohort, hence should ideally be determined in each population. Serum HER-2/neu assay though non invasive and cost efficient, may not yet be considered as an alternative or complementary marker to tissue HER-2/neu at this time.
Keywords: Serum Her-2/Neu, Reference Intervals, Multiethnic
Cite this paper: Alicezah MK , Fathimah M , M Thevarajah , M Naicker , Yip CH , Taib NA , Rozita AM , Serum HER-2/neu Reference Intervals in a Multiethnic Malaysian Population, Clinical Medicine and Diagnostics, Vol. 3 No. 1, 2013, pp. 1-5. doi: 10.5923/j.cmd.20130301.01.
Article Outline
1. Introduction
- Breast cancer or malignant breast neoplasm is the commonest cancer in women aged 15-49 years in Malaysia[1]. It is the leading cause of cancer death among women of all ethnic and age groups in Malaysia[1]. HER-2/neu positive breast cancers tend to be more aggressive than other types of breast cancer. The overexpression of HER-2/neu on primary breast cancer has been associated with poor patient prognosis and an overall decreased survival[2]. Tumors with overexpression or amplification of HER-2/neu can be successfully treated with trastuzumab[3,4]. HER-2/neu is a human epidermal growth factor receptor-2 oncogene that is important for cell proliferation in breast cancer patients. It is shed from cancer cells into the blood stream and it can be detected about 18% with primary breast cancer and over 50% in metastatic breast cancer[5,6].Traditionally, HER-2 / neu status is assessed by immunohistochemistry (IHC) and fluorescence in situ hybridisation (FISH). IHC is used to detect HER-2/neu protein levels by the degree of tissue staining which is subjectively scored from 0 (negative) to 3+[7] FISH method has greater accuracy which detects the amount of genetic material present in the tissue, however the technique requires a high degree of technical expertise and it is costly[8]. Both methods require tissue sampling which is an invasive technique. A new alternative diagnostic marker now exists in the form of an immunoassay which is targeted towards the circulating extracellular domain (ECD) of HER-2/neu in blood specimens[8]. The ability to assess a patient’s HER-2/neu status from blood samples offers a dynamic test which can be performed at any time before or after surgery. Therefore, a considerable impetus exists to better define serum HER-2/neu concentration. In this study, we established reference range in 3 group of population (breast cancer, BBD and healthy volunteers (control group). We also reviewed the need to establish a different reference range in different ethnic group in Malaysia among Malay, Chinese and Indian. Apart from that, we evaluated the association of serum HER-2/neu concentration with age, menopausal status, races, tumor histology, grade, ethnic group and tissue receptor status (estrogen receptor,ER and progesterone receptor,PgR) and tissue HER-2/neu status. We also reviewed the correlation between serum HER-2/neu with tissue HER-2/neu.
2. Materials and Method
2.1. Patient Population and Study Design
- This is cross sectional study conducted by the Department of Pathology, Surgery and Oncology, University Malaya Medical Centre (UMMC) from July 2010 until January 2011. A total of 40 patients of newly diagnosed breast cancer were selected from those with biopsy proven ductal carcinoma and subjected for mastectomy. A preoperative (baseline) blood sample was collected a day prior to surgery which was processed at the Division of Laboratory Medicine, UMMC. The tissue specimens were sent to histopathology laboratory UMMC for histopathological examination (HPE). Patients with recurrent breast cancer or breast cancer on therapy (neoadjuvant therapy) were excluded from the study. In addition, 40 patients of benign breast cancer (BBD) including fibroadenoma and breast cyst were recruited in this study. The patients were selected among those who were subjected for lumpectomy. As per breast cancer patients, a baseline blood test was taken prior surgery. A group of 102 healthy volunteers were arbitrarily selected among the UMMC staff. Data on patient’s age, menopausal status, site of lesion, ethnicity and histopathological report including grade of tumor, receptor status (ER, PR, HER-2/neu) were collected from patient case file. This study was approved by the medical ethics institution, University Malaya Medical Centre and the patients had signed informed consent on recruitment.
2.2. Biochemical Analysis
- The blood samples were collected into a plain tube which was immediately centrifuged to separate the serum and stored at-20℃ until analysis. Serum Her-2/neu concentrations were measured by a two site sandwich immunoassay using direct, chemiluminescence technology on the fully automated analyser, (ADVIA Centaur, USA). The lite Reagent is composed of the monoclonal mouse antibody, TA-1, labelled with acridinium ester. The Fluorescein Conjugate Reagent is composed of the monoclonal mouse antibody, NB-3, labelled with fluorescein. A direct relationship exists between the amount of HER-2 present in the patient sample and the amount of relative light units (RLUs) detected by the system. Two levels of control serum were assayed daily for quality control measurement. The within run precision had a coefficient variation (CV) of 6% for low level and 10.5% for high level control respectively. The between run precision had a total CV of 9.6% while the within run precision CV ranged from 6.7% to 6.9%. The total CV claimed by anufacturer had a range of 3.2-5.7 %. , while the within run CV was under 5.6%.
2.3. Immunohistochemistry Analysis
2.3.1. Tissue HER-2/neu analysis
- Immunohistochemistry staining of specimens was carried out on formalin- fixed paraffin embedded breast cancer tissue using Polyclonal Rabbit Anti- Human c-erbB-2 Oncoprotein(Code A0485; DakoCytomation Denmark A/S). Scoring of tissues HER 2/neu was based on the Hercep Test criteria. The immunoreaction was scored as ; 3+ if >10% of tumour cells showed strong and complete membrane staining, 2+ if membrane positivity was moderate and complete in > 10% cells, 1+ if membrane positivity was weak and incomplete in 10% cells and if membrane staining was absent or present in < 10% of cell. Tissue HER-2/neu was considered positive when the tumour scored as 3+ or 2+. Otherwise, tissue HER-2/neu was considered negative when the tumour scored as 1+/ 0.
2.3.2. Estrogen and Progesterone Receptor Status Analysis
- Estrogen(ER) and progesterone receptor (PgR) status were determined via standard immunohistochemistry on archived formalin-fixed, paraffin-embedded tissue. ER was detected using rabbit monoclonal antibody, clone SP1; (diluted 1:100-1:400, NeoMarkers For Lab Vision Corporation). PgR was detected using monoclonal mouse anti human progesterone receptor antibody; PgR636 (diluted 1:600, NeoMarkers For Lab Vision Corporation). Positive immunostaining was recorded for ER and progesterone receptor as nuclear staining: 1+ (10% of cells positive), 2+ (11% to 50% positive), and 3+ (greater than 50% positive). Appropriate positive and negative controls were tested simultaneously with the test slidesSlides were independently interpreted by multiple surgical pathology residents with an experienced pathologist on call.
2.3.3. Statistical Analysis
- Data were analysed by using Statistical Package for Social Sciences (SPSS) /version 18.0 software for Windows. The reference range was established as mean + 2 SD. The mean of each data set (breast cancer, BBD and healthy volunteers) and mean of each ethnic group (Malay, Indian, and Chinese) were evaluated by analysis of variance (ANOVA. The X2 test was used to assess the association between serum HER-2 neu concentration with clinicopathological parameters and tissue HER-2/neu. The p value <0.05 was considered statistically significant.
3. Results
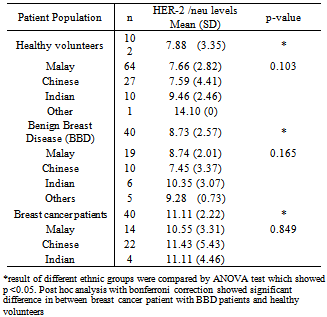
- Table 1 summarized the mean (SD) of serum HER-2/neu concentration in the healthy volunteers (n=102), BBD (n=40) and the breast cancer patients (n=40). Analysis of these means by ANOVA test showed significant differences in between the 3 data set means (p<0.05). The post hoc analysis with bonferroni correction showed a significant difference between breast cancer patients with healthy volunteer and BBD patients. However, no significant difference was noted between the BBD patients with healthy volunteers (p >0.05). The serum HER-2/ neu concentration of the multi ethnic Malays, Chinese, Indian and other native minority healthy volunteers, BBD and the breast cancer patients are seen in Table 1. No significant difference between each ethnic populations, (p >0.05) were found by ANOVA analysis in the 3 different populations showed.
|
|
|
|
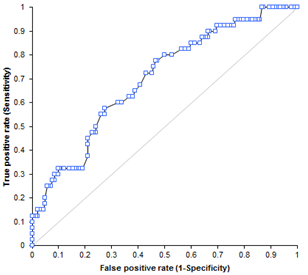 | Figure 1. Receiver Operating Curve (ROC) of serum HER-2/neu concentration in 40 patients with primary breast cancer |
4. Discussion & Conclusions
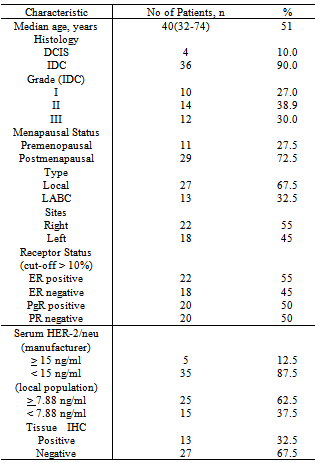
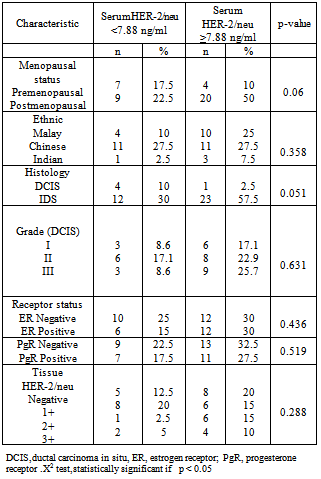
- The demographic data of breast cancer patients shows that the median age was 51 years which was younger compared to previously published studies[9,10]. A younger median age of 44 years for breast cancer also reported in a similar study done in Korean population[11]. In this study, it was shown that amongst the different ethnic groups in Malaysia, the Chinese had a higher incidence of breast cancer. This result was supported by data from the Second report of the National Cancer Registry and Penang Cancer Registry which had reported a similar higher incidence of breast cancer among Malaysian Chinese[12, 13]. Similarly, in our neighbouring country, Singapore; it had been reported that the Chinese ethnic group was at higher risk to develop breast cancer compared to the Malays and Indians[14]. The mean of serum HER-2/neu concentration in breast cancer patients was the highest followed by the benign breast disease patients, and the healthy control group. This result is expected as about 18% of primary breast cancer can have elevated serum HER-2/neu. In terms of HER-2/neu gene amplification, it is noted that about 20-30% of breast cancer patients can have this protein overexpression[15,16,18]. Malaysian is a multiracial population which is dominated by Malays, Chinese and Indians. By taking account of the multhiethnicity of the Malaysian population, we tried to established reference range for each ethnic group. However, we did not find any significant difference among the ethnic group, thus we conclude that a single reference range can be used for all ethnic groups of Malaysian.The cut off value of serum HER-2 at 7.88ng/ml was found to be lower when compared to the recommended manufacturer (Siemens) kit insert at 15 ng/ml[15].. We attributed the low cut off value could be related to geographical significance as we noted from other similar work done on the Korean population also yielded lower cut off value of 10.2 ng/ml[11].In other studies, there was a correlation between serum HER-2/neu with clinicopathological parameters[17,19,21] . However, in this study, no significant correlation was established. Possible contributions to the negative correlation could be due to the relatively small sample size that caused statistically no significance result. Few studies have evaluated the association between tissue HER-2/neu status (IHC) and baseline serum HER-2/neu levels[20]. The targeted molecular domains and protein function are different from the IHC method recognizes intracytoplasmic domains of the entire HER-2/neu receptor, while the serum HER-2/neu assay measures the extracellular domain (ECD) of the HER-2/neu receptor by using two mAbs recognizing two independent epitopes[22]. However, in this study, the serum HER-2/neu concentration did not show significant correlation with tissue HER-2/neu. In conclusion, the clinical utility of the serum HER-2 neu is still lacking and more research is needed to gather more information regarding this marker. Serum HER-2/neu assay cannot yet be considered as an alternative or complementary marker for tissue HER-2/neu.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML