-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2011; 1(1): 21-27
doi: 10.5923/j.cmd.20110101.04
Comparison of an Immunochromatographic Rapid Strip Test, ELISA and PCR in the Diagnosis of Hepatitis C in HIV Patients in Hospital Settings in Cameroon
Tebit E. Kwenti 1, Richard Njouom 2, Longdoh A. Njunda 1, Henri Lucien F. Kamga 1
1Department of Medical Laboratory Sciences, Faculty of Health Sciences, University of Buea, Buea, Box 63, Cameroon
22Laboratoire de Virologie, Centre Pasteur du Cameroon, Yaounde, Box 1274, Cameroon
Correspondence to: Tebit E. Kwenti , Department of Medical Laboratory Sciences, Faculty of Health Sciences, University of Buea, Buea, Box 63, Cameroon.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Cameroon belongs to the group of countries highly endemic for hepatitis C viruses. Coinfection of hepatitis C and HIV are also common due to the shared route of transmission of both viruses. In hospital settings in Cameroon, diagnosis prior to treatment of hepatitis C is based solely on the results obtained with an immunochromatographic rapid strip test (97%). This study was aimed at determining the validity of the results that is obtained when an immunochromatographic rapid strip test is used to diagnose hepatitis C virus infection in HIV-positive patients in comparison with more sensitive and specific methods like ELISA and PCR. In a cross-sectional study in two parts, 700 participants were enrolled, 350 were HIV-positive patients and a control group of 350 individuals not infected with HIV. All participants were screened for anti-HCV antibodies using ACON HCV strip test, an assay commonly used in 57·1% of Cameroon hospitals. While using the rapid strip test, of the 350 HIV-positive patients, 25 (7·1%) were found to be positive with the rapid strip test of whom 3(12%) were positive with an ELISA and all 3(100%) positive with the ELISA were also positive with PCR. Evaluation of the rate of false positives with the rapid strip test using ELISA as the gold standard gave a rate of 6·3%. Meanwhile in the control group, after screening with the rapid strip test, 39 (11·1%) were positive of whom 6 (15·4%) were positive with the ELISA and 3 (50%) of the 6 positive with the ELISA were positive with the PCR. Evaluation of the rate of false positives with the rapid strip test in the control group using ELISA as the gold gave a rate of 9·6%. False positive results with this immunochromatographic rapid strip test for the diagnosis of hepatitis C virus infection is therefore common and therefore reinforce the need for a confirmatory test prior to treatment in hospital settings in Cameroon.
Keywords: Hepatitis C Virus, HIV, Immunochromatographic Rapid Strip Test, ELISA, PCR
Cite this paper: Tebit E. Kwenti , Richard Njouom , Longdoh A. Njunda , Henri Lucien F. Kamga , "Comparison of an Immunochromatographic Rapid Strip Test, ELISA and PCR in the Diagnosis of Hepatitis C in HIV Patients in Hospital Settings in Cameroon", Clinical Medicine and Diagnostics, Vol. 1 No. 1, 2011, pp. 21-27. doi: 10.5923/j.cmd.20110101.04.
Article Outline
1. Introduction
- Coinfection with HIV and Hepatitis C virus is a major public health problem in both developed and developing countries. A good proportion, 4–5 millions of the 40 millions HIV patients are coinfected with Hepatitis C virus[1,2]. This is probably because both viruses are acquired through similar parenteral route. Hepatitis C virus infection is the major cause of liver disease (chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma) and its natural history in HIV is accelerated[3].In hospital settings in Cameroon, diagnosis of hepatitis C is often done using immunochromatographic rapid strip tests. These assays work on the common principle ofantibody present in the test serum/plasma reacting with protein coated particle (protein A) and migrating upward on amembrane chromatographically by capillary action to react with recombinant HCV antigen present on the membrane thereby generating a coloured line in the test region. So much reliance is placed on these immunoassays because they are cheap and easily affordable, they require minimum technical expertise to operate, and also very rapid to give results especially in a setting where diagnosis has to be made before treatment commences on daily bases. Treatment is with pegylated interferon and ribavirin combination therapy. Side effects due to these regiments are very common which include hematologic side effects (in most cases anemia)[4] and the possibility of drug interaction with anti-HIV drugs. The immunologic reaction of patients infected with Hepatitis C virus is diverse especially duringseroconversion phase thereby giving varying results with these immunochromatographic rapid strip tests. The colour intensity of the test line will depend on the concentration of antibody to hepatitis C produced by the patients. Therefore in the presence of immunodeficiency like HIV where adequate antibody production may be a problem, this may have an effect on the quality of the results that is produced by these rapid test strips. This cross-sectional study in two parts therefore seeks to determine the validity of the results obtain with an immunochromatographic rapid strip test when used to diagnose hepatitis C in HIV patients in comparison to more accurate diagnostic methods (ELISA and PCR) in today’s health care practice and also to determine the risk factors involved for the transmission of hepatitis C virus.
2. Materials and Methods
2.1. Hospital Survey
- Hospitals were randomly selected in the country and a questionnaire was administered to them in order to determine how hepatitis C virus infection is being diagnosed.
2.2. Study Area
- This study was done in Bamenda using two facilities; Mbingo Baptist Hospital (MBH) and Nkwen Baptist Health Center. Bamenda (coordinates 5°56′N 10°10′E) is the capital of the North West Region of Cameroon (a country situated on the west wing of Central Africa). The region was chosen because of it great cultural diversity and the relatively high prevalence of HIV (6.9%), more than any other region in the country[5].
2.3. Study Population
- This study was approved by the Cameroon Baptist Convention Institutional Review Board (CBC IRB). Blood samples were collected from 700 randomly selected participants after getting their informed consent between 20 June and 01 August 2011. These participants included 350 HIV positive patients and a control group (HIV-negative) of 350 participants. Among the 350 HIV-positive patients were 207 (73·4%) women and 93 (26·6%) men (median age of 37years) meanwhile 245 (70%) women and 105 (30%) men made up the control group (median age of 35years). The inclusion criterion was limited to individuals above 18 years because of the sexually sensitive nature of the questionnaire they had to fill in order to determine risk factors for the transmission of Hepatitis C virus in the region. The questionnaire was duly explained to the participants in the local pidgin language after which 5ml of blood was collected into two separate tubes, EDTA tube and dry tube for every participant. The tubes were centrifuged and the serum from the dry tubes were transferred into eppendorf tubes and frozen at -20˚C until further analysis, meanwhile the plasma samples were used for the screening process. Questionnaires and samples were identified only by a study number.
2.4. Screening for HIV
- Screening for HIV was done on the control group to confirm their HIV-negative status. This was done in accordance with the Cameroon algorithm for HIV screening approved by the WHO. The first line test was Determine™ HIV (Abbott Laboratories, Abbott Park, IL, USA) and the second line test was Hexagon (Human Diagnostic, Germany).
2.5. Screening for HCV
- Initial screening for anti-HCV antibodies was done using an immunochromatographic rapid strip test, ACON HCV rapid test (ACON Laboratories, Inc) on the plasma of the samples collected. The manufacturer’s instructions were closely followed.Positive samples with the rapid strip test were taken to the virology laboratory of Centre Pasteur in Yaounde for confirmation.
2.6. Confirmation of Anti-HCV Antibodies
- The samples that were positive for anti-HCV antibodies with the rapid strip tests were confirmed for anti-HCV an-tibodies using a commercial third-generation ELISA, MONOLISA anti-HCV plus version 2 (Bio-Rad, Marne La Coquette, France) paying close attention to the manufac-turer’s instructions. The results of the assay were expressed as a ratio (R) of the optical density (OD) of the sample to the calculated cut-off absorbance as recommended by the manufacturer. Samples were considered positive with a ratio (R) ≥ 6·0.
2.7. HCV RNA Detection
- The samples that were positive with the ELISA were further processed to detect the presence of HCV RNA. Viral RNA was extracted from 140µl of serum with a Qiamp® viral mini kit (Qiagen, Courtaboeuf, France) according to the manufacturer’s instructions. The extracted RNA was used as a template and amplified using an in-house RT-PCR with primers to the NS5B region (Pr3, and Pr 2). The amplified products were analysed by electrophoresis in a 1.5% agarose gel.
2.8. Statistical Analysis
- Statistical analyses were performed with the MINITAB 15 English. Differences between proportions were determined using the chi-square (χ²) or the Fisher’s exact test. P values < 0·05 were consider to be statistically sensitive and represents 95% of the population.
2.9. Limitations
- ELISA and PCR could not be performed on samples from all the 700 participants because of cost. They were performed only on samples that were positive with the immunochromatographic rapid strip test. In this study only the rate of false positives will be evaluated. It is therefore assumed that all negative results with the rapid strip test are negative with ELISA and PCR.
3. Results
- 68 (97%) of the 70 hospital laboratories that were considered in the survey, are currently using immunochromatographic rapid strip tests as the sole diagnostic criteria for infection with hepatitis C virus. Two (3%) hospitals use an ELISA to diagnose hepatitis C virus infection but none of the hospital that were included in the survey use PCR to confirm the infection. The most common type of rapid strip test is ACON® HCV strip test which was currently in use by 40 (57·1%) of the 70 hospitals in the survey use (Table 1). Base on the record obtained from the HIV-positive patients, 280 (80%) of the 350 patients were on antiretroviral therapy. The most common HIV type among the 350 HIV-positive patients were HIV-1 where 275 (78%) were infected, follow by HIV-1&2 where 48 (14%) were infected and HIV-2 where 27 (8%) were infected with the virus (Figure 1). The individuals of the control group were confirmed not to be infected with HIV.
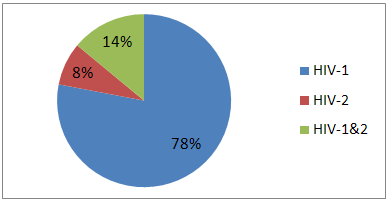 | Figure 1. The distribution of the HIV types among the HIV-positive participants. |
|
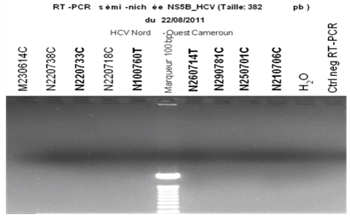 | Figure 2. HCV RNA bands on agarose gel electrophoresis. The labels in bold are the samples from which RNA was detectable. |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
4. Discussion
- From the survey of hospitals in Cameroon, it was observed that a good number of hospital (97%) diagnose hepatitis C using a rapid strip test, only a few (3%) use ELISA and none (0·0%) use PCR. The most common rapid strip test is the ACON® HCV strip test which was in use by 57·1% of hospitals to diagnose hepatitis C virus infection.This study therefore shows the discrepancy that exists when the diagnosis of hepatitis C virus is made solely on the results obtained from immunochromatographic rapid strip tests. Of the 25 samples from the HIV-positive group, only 3 (12%) were positive with the ELISA and a similar scenario was observed in the control group where out of the 39 samples that were positive with the same rapid strip test, only 6(15·4%) were positive with the ELISA. The Monolisa anti-HCV ELISA used in this study is a third-generation ELISA and has been shown to be very sensitive (100%) and specific (98%) and even has the capability of reducing the window period for detection of the virus by 72 days[6]. It is therefore very likely that a positive result with ELISA will also be positive with PCR as shown by the observation that all the 3 (100%) samples that were positive with the ELISA from the HIV-positive group were also positive with PCR and 3 (50%) of the 6 samples from the control group that were positive with the ELISA were also positive with PCR. Evaluation of the rate of false positives with the rapid strip test in the HIV-positive group when compared with ELISA gave a rate of 6·3%. This rate when stacked close to the rate (9·4) obtained in the control group is found to be lower. However this difference was not significant statistically (χ² = 2·040, P = 0·1532). Only the rate of false positives was evaluated in this study and not the rate of false negatives because this study was designed to determine the validity of the results that are given out in the hospitals in Cameroon as positive in which treatment begins almost immediately with therapy that has a lot of side effects on the patients and which is also very costly for the patients. However, false negative results can be produced in the case of HIV patients who may have difficulties in raising antibodies against the virus. A false negative result obtain in this case will be better than a false positive result. On the contrary, in blood banking, a false negative result can be disastrous because infected blood will be transfused thereby transmitting the virus to an uninfected individual. The observation that in the control group, HCV RNA could be detected in only 3(50%) of the 6 samples that were positive with the ELISA can either signify a false positive, or the phenomenon known as spontaneous viral clearance, which is in accordance with the natural history of infection with hepatitis C virus whereby 10-60% of individuals that have been infected with the virus, have the ability to clear the virus from their system even without treatment[7]. This phenomenon further reinforces the importance for investigating further before beginning treatment. False positives with ELISA cannot be completely ruled out in Africa where studies have shown that Africans produce antibodies that react non-specifically with ELISAs and have even prompted the development of newer generations of ELISA[8-10].
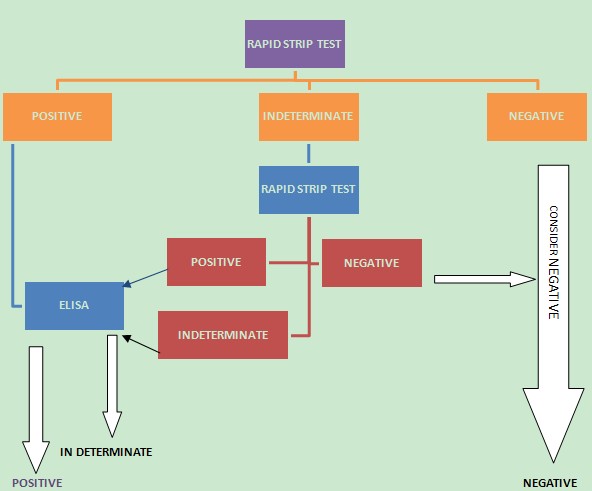 | Figure 3. A propose algorithm for diagnosis of HCV in hospital settings in Cameroon |
5. Conclusions
- A positive result for anti-HCV antibodies gotten with an immuno-chromatographic rapid strip test does not warrants that treatment should begin. False positive results are common. Therefore the presence of the disease should be investigated further using a more sensitive and specific assay prior to treatment. Although PCR assays are very expensive to be incorporated into hospital setting in Cameroon, an ELISA which is less expensive and more affordable can be implemented to give more valid results. A negative result too does not exclude the presence of the infection. If symptoms persist, then the infection should be investigated further with a PCR assay. It is important that diagnosis should be done together with the patient medical history since the major risk factors for infection with the virus in the North West Region of Cameroon is the age of the individual. Despite the relative high prevalence of HIV in the North West region, the seroprevalence of coinfection with HCV among HIV patients is low (0·9%) owing to the different demographic nature of both diseases and being HIV-positive is therefore not a major risk factor for infection with hepatitis C virus.
ACKNOWLEDGEMENTS
- This study was funded by Mr Tebit Mudoh Thomas, Mrs Tebit Mamo, Mr Che Venatus Sanigong and Mrs Medjuine Beatrice. Writing assistance by Dr. Julius Atashili is deeply appreciated. We extend our sincere gratitude to the staff of FHS (UB), Centre Pasteur du Cameroun, Mbingo Baptist Hospital and Nkwen Baptist Health Center.
References
| [1] | Rockstroh, J. K., and Spengler, U., 2004, HIV and hepatitis C virus coinfection., Lancet of Infectious Diseases, 4,437-44 |
| [2] | Alter, M. J., 2006, Epidemiology of viral hepatitis and HIV coinfection., Journal of Hepatology, 44 (1 suppl), S6-9 |
| [3] | Piliero, P. G., and Faragon, J. J., 2002, Case report. Hepatitis C virus and HIV coinfection., AIDS Read, 12,443-4, 448-51 |
| [4] | Nachnani, J. S., Rao, G. A., Bulchandani, D., Pandya, P. K., Alba, L. M., 2010, Predictors of hematological abnormalities in patients with chronic hepatitis C treated with interferon and ribavirin., Annals of hematology, 89(2),121-5 |
| [5] | UNAIDS., 2010, A global view of HIV infection, Cameroon |
| [6] | Alados-Arboledas, J. C., Calbo-Torrecillas, L., Lopez-Prieto, M. D., de Francisco-Ramirez, J. L., and de Miquel-Sastre, C., 2007, Clinical assessment of Monolisa HCV Ag-Ab ULTRA (Bio-Rad) in a general hospital., Enfermedades Infecciosas y Microbiologίa Clίnica, 25(3),172-6 |
| [7] | Caruntu, F. A., and Benea, L., 2006, Acute hepatitis C virus infection: Diagnosis, pathogenesis, treatment., Journal of Gastrointestinal and Liver Diseases, 15 (3), 249–56 |
| [8] | Wong, D. C., Diwan, A. R., Rosen, L., Gerin, J. L., Johnson, E. G., Polito, A. and Purcell, A., 1990, Non-specificity of anti-HCV test for seroepidermiology analysis., Lancet, 336, 750-751 |
| [9] | Desmyter, J., Goubau, P., Vermylein, C. and Sondag-Thall, D., 1991, Ortho anti-HCV clinical trials in Belgium: selected results. In: viral Hepatitis C, D and E, Shikata T, Purcell RH and Uchida T (editors)., Amsterdam: Elsevier, PP. 87-90 |
| [10] | Raghuram, S., Subramaniam, T., Daniel, D., Sridharan, G., and Abraham, P., 2003. Occurrence of false positives during testing for antibodies to hepatitis C virus among volunteer blood donors in India., Journal of Clinical Microbiology, 41,1788-1790 |
| [11] | Njouom, R., Tejiokem, M. C., Zanga, M. C., Pouillot, R., Ayouba, A., Pasquier, C., and Nerrienet, E., 2006, A cost-effective algorithm for the diagnosis of hepatitis C virus infection and prediction of HCV viraemia in Cameroon., Journal of Virological Methods, 133(2), 223-226 |
| [12] | Ndjomou, J., Kupfer, B., Kochan, B., Zekeng, L., Kaptue, L., and Matz, B., 2002, Hepatitis C virus infection and genotypes among human immunodeficiency virus high-risk groups in Cameroon., Journal of Medical Virology, 66(2),179-86 |
| [13] | Laurent, C., Bourgeois, A., Mpoudi, M., Butel, C., Mpoudi-Ngolé, E., and Delaporte, E., 2007, HIV and hepatitis C virus coinfection Cameroon., Emerging Infectious Diseases, 13(3),514-6 |
| [14] | Pépin, J., Lavoie, M., Pybus, O. G., Pouillot, R., Foupouapouognigni, Y., Rousset, D., Labbé, A. C., and Njouom, R., 2010, Risk factors for hepatitis C virus transmission in Collonial Cameroon., Clinical Infectious Disease, 51(7),768-776 |
| [15] | Kowo, M. P., Goubau, P., Ndam, N. E-C., Njoya, O., Sasaki, S., Seghers, V., and Kesteloot, H., 1995, Prevalence of hepatitis C virus and other blood-borne viruses in pygmies and neighbouring Bantus in Southern Cameroon., Transactions of the royal Society of Tropical Medicine and Hygiene, 89,484-486 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML