-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2021; 11(2): 36-42
doi:10.5923/j.cmaterials.20211102.02
Received: Sep. 29, 2021; Accepted: Oct. 15, 2021; Published: Oct. 30, 2021

Superabsorbent Hydrogel derived from Lemon Juice/Ethylenediamine with Maleic Acid as a Cross-Linker
Titus M. Kasimu1, Harun M. Mbuvi1, Francis M. Maingi2
1Department of Chemistry, Kenyatta University, Nairobi, Kenya
2Department of Science Technology and Engineering, Kibabii University, Bungoma, Kenya
Correspondence to: Francis M. Maingi, Department of Science Technology and Engineering, Kibabii University, Bungoma, Kenya.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Hydrogels are 3-dimensional polymer network materials with the ability to absorb a large amount of water. The current advancement in technology has increased their demand in the fields of industrial and environmental applications. This study reports the synthesis and characterization of superabsorbent hydrogels derived from lemon juice. The preparation involved linking lemon juice (LJ) with Ethylenediamine (EDA) via an amide linkage to obtain HLE-1 hydrogel. The polymer hydrogel was then cross-linked with maleic acid via an ester linkage to form HLE-2 hydrogel. Characterization was done using FT-IR, SEM, and XRD. The optimization of the swelling conditions was studied by varying contact time and the dosage of both lemon juice and the cross-linker. XRD analysis showed the conversion of amorphous hydrogel HLE-1 to crystalline hydrogel HLE-2 upon cross-linking. The FT-IR spectra showed a new strong symmetric stretching -COO- peak at 1079.83 cm-1 in HLE-2 indicating successful ester linkage. SEM analysis showed pores of different sizes and shapes in HAE-2 compared to a rigid, concrete, and smooth surface in HLE-1. Upon the optimization of the synthesis conditions of the cross-linked hydrogel, a swelling capacity of 925% was obtained. Crosslinking the hydrogel improved its water absorption ability. The high swelling capacity of the hydrogel provides a baseline for potential application in agriculture, especially in semi and arid regions.
Keywords: Characterization, Lemon juice, Cross-linking, Ethylenediamine, Superabsorbent hydrogel
Cite this paper: Titus M. Kasimu, Harun M. Mbuvi, Francis M. Maingi, Superabsorbent Hydrogel derived from Lemon Juice/Ethylenediamine with Maleic Acid as a Cross-Linker, International Journal of Composite Materials, Vol. 11 No. 2, 2021, pp. 36-42. doi: 10.5923/j.cmaterials.20211102.02.
Article Outline
1. Introduction
- Polymer superabsorbent hydrogels are three-dimensional hydrophilic structures that are prepared through the linking of materials either chemically or physically to form copolymers with super absorbing characteristics. The hydrophilic groups in hydrogels polymer backbone (–CONH2, –OH, –COOH, –SO3H) have the ability to absorb water many times the original weight while maintaining their structures [1]. The recent rapid increase in population growth has led to the development of new technologies of synthesizing hydrogels towards major applications in wastewater treatment, agriculture, and food industry, biotechnological and medical fields. Researchers have drawn interest in this field due to the increasing demand for hydrogels [2]. Permanent and reversible hydrogels depend on the nature of cross-linkers in the network of polymer [3]. Reversible hydrogel has a non-homogeneous network as a result of non-covalent bonds and temporary chain entanglements [4]. Currently, most hydrogels in literature have been developed in the laboratory with their large-scale production being costly, toxic, complex in synthesis, and non-biodegradable, with minimal reports in Africa on their use 1], [5]. The availability of lemon juice coupled with its rich carboxylic groups makes it a potential monomer in hydrogel synthesis [6]. On the other hand, lemon juice incorporated with ethylenediamine organic surfactant forms polymer gel which when cross-linked with maleic acid can form crystalline phases with small particle size and large surface area for water absorption. This study aimed at synthesizing and characterizing a green superabsorbent hydrogel from bio-derived materials to try and address the technical limitations of the existing hydrogels.
2. Materials and Methods
2.1. Reagents and Chemicals
- The lemons were obtained from the local market in Machakos County - Kenya then transported to Kenyatta University laboratory. Ethylenediamine was obtained from Merck Chemical Co (Darmstadt, Germany), and maleic acid was purchased from Kenya science chemical limited Kenya.
2.2. Extraction of Lemon Juice from Lemon Fruits
- During the extraction of lemon juice, the peels of lemon fruits were removed to obtain the inner part. The fresh soft parts of the fruit were cut into smaller sizes, put into an electric blender machine for blending. After blending the mixture was then filtered using a standard sieve of 14-16 micropores to obtain lemon juice [7].
2.3. Preparation of Hydrogel from Lemon Juice and Ethylenediamine (HLE-1)
- 145.0mL of lemon juice and 90.0mL of ethylenediamine (14.8M) were measured and put in an aluminum container. 100 mL of distilled water was added to the mixture to aid in the solvation process. The mixture was then heated at a temperature of 373 K to 405 K in an oven to form a viscous gel. The gel was allowed to cool to form solid hydrogel HLE-1.
2.4. Preparation of Cross-Linked Hydrogels (HLE-2)
- The cross-linked HLE-2 superabsorbent hydrogel was prepared by adding 75.0mL of 3.5M maleic acid to HLE-1 hydrogel. The mixture was stirred and heated continuously at a temperature of 373K to 405K to form a viscous gel. It was then cooled to form HLE-2 hydrogel. Figure 1 shows the preparation scheme of HLE-2 hydrogel.
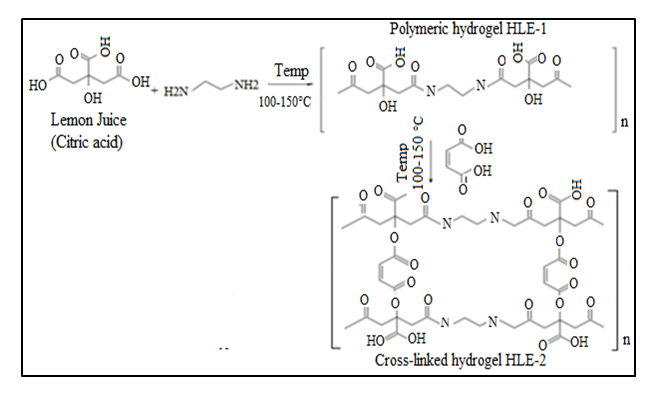 | Figure 1. Scheme of preparation of HLE-2 hydrogel |
2.5. Characterization of the Hydrogel
2.5.1. Fourier Transform Infrared (FT-IR) Analysis of the Superabsorbent Hydrogels
- The functional group analysis of the uncross-linked HLE-1 and cross-linked HLE-2 was done using FT-IR spectroscopy [8]. The obtained crystal samples were dried at a temperature of 305K in an oven for 6 hours until a constant weight was attained. The dry sample of each hydrogel HLE-1 and HLE-2 were mixed with pre-dried KBr in a ratio of 25: 1 mg respectively. The fine homogenous mixture was compressed to form a transparent pellet then analyzed using the transmission method at 4000-200 cm-1 wavelength range (Shimadzu IR Tracer-100) [9].
2.5.2. Phase Composition Analysis of the Superabsorbent Hydrogels
- The XRD analysis procedure for phase composition of the HLE-1 and HLE-2 hydrogels was adopted from Aikawa et al. [10]. The samples of superabsorbent hydrogels were cut into smaller sizes of 1 cm2 followed by drying the samples at a temperature of 305K for 6 hours. The dry samples were placed on a silicon wafer, the measurement of phase and morphology of samples taken continuously from scattering angle (2θ) ranging 10 to 90° at 40 kV and a current of 15 mA with Cu ka radiation (1.54059-1.54441) using XRD (model Hossein Beygin German).
2.5.3. Microstructural Analysis of the Superabsorbent Hydrogels
- The superabsorbent hydrogel microstructures of HLE-1 and HLE-2 were determined using a (ZEISS SUPRA 60 German) bright microscope at an accelerating voltage of 250 kV. The prepared samples were dried in the oven at 45°C for 6 hours until a constant weight was attained followed by coating with gold then placing them in an enclosed glass slide to obtain micrographs [11].
2.6. Effect of Lemon Juice (LJ) Dosage on Swelling Capacity of Cross-Linked Hydrogel
- The hydrogels were prepared by varying dosage of lemon juice (LJ) from (0.9, 1.8, 3.6, 5.4, 7.2, and 9.0mL) while maintaining the volume of ethylenediamine and maleic acid as 4.5mL and 5.0mL respectively. 2.0g of each prepared sample gel was put in a uniform polyester bag and immersed in water for 24 hours to determine the swelling capacity.
2.7. Effect of Maleic Acid Dosage on the Swelling Capacity of the Hydrogel
- The effect of the maleic acid (cross-linker) concentration was studied by varying dosages (1.25, 2.5, 3.75, 5.0, 6.25, and 7.5mL) while maintaining the volume of lemon juice at 7.2mL, and that of ethylenediamine as 4.5mL. 2.0 g of each gel sample weighed accurately was then immersed in water for 24 hours to determine the swelling capacity.
2.8. Equilibrium Water Content (EWC)
- The swelling percentage capacity of HLE-2 samples was determined gravimetrically in deionized water at 25°C using a polyester bag method [12], [13], [14]. The prepared samples were allowed to dry to a constant weight in an oven at 45°C. 2.0 g of the dried sample was put in a polyester bag made of 200 mesh nylon and then immersed in the water to swell. The sample was then removed from the water and excess water was removed using Whatman filter paper. The equilibrium swelling capacity of the hydrogel was determined using equation 1 13, [15].
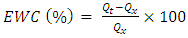 | (1) |
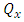 is the initial weight of hydrogel before swelling and
is the initial weight of hydrogel before swelling and 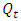 is the final mass of the swollen hydrogel.
is the final mass of the swollen hydrogel.2.9. The Effect of Contact Time on the Swelling Capacity of the Hydrogel
- The procedure of studying the effect of contact time on the swelling capacity of HLE-2 was adopted from Takigami et al. [16]. Varying contact times in the range (0.5, 1, 2, 4, 6, 12, and 24 hours) were used to study the effect of contact time on swelling capacity. 2.0 g of HLE-2 hydrogel was immersed in 500 mL of distilled water for a specified contact time. The samples were then removed periodically after the expiry of the specified time and new mass determined. The swelling capacity was then determined using equation 1 provided in section 2.8.
3. Results and Discussion
3.1. FT-IR Spectrum of Superabsorbent Hydrogels
- FT-IR was used to determine the functional groups present in the green superabsorbent hydrogel before and after cross-linking with maleic acid. Figure 2 shows the IR spectrum of the hydrogel before crosslinking. The hydrogel was coded as HLE-1.
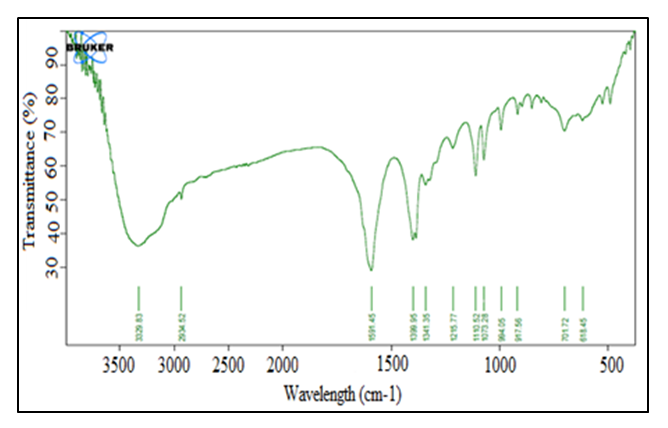 | Figure 2. FT-IR spectrum of HLE-1 hydrogel |
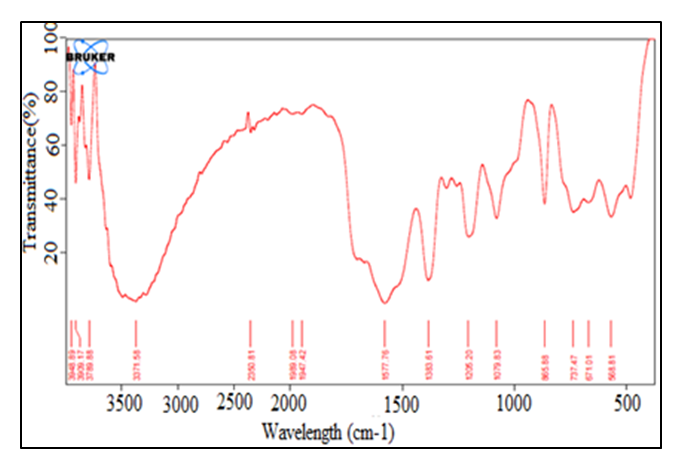 | Figure 3. FT-IR spectrum of HLE-2 hydrogel |
3.2. Phase Composition of HLE-1 and HLE-2 Hydrogels
- The phase composition of the samples was determined using the XRD technique. The figure 4 and 5 represents the diffraction patterns of HLE-1 and HLE-2 hydrogels respectively.
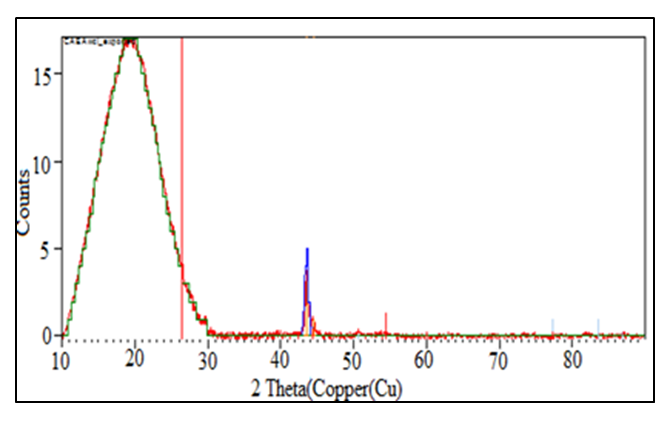 | Figure 4. Powdered diffraction pattern of HLE-1 hydrogel |
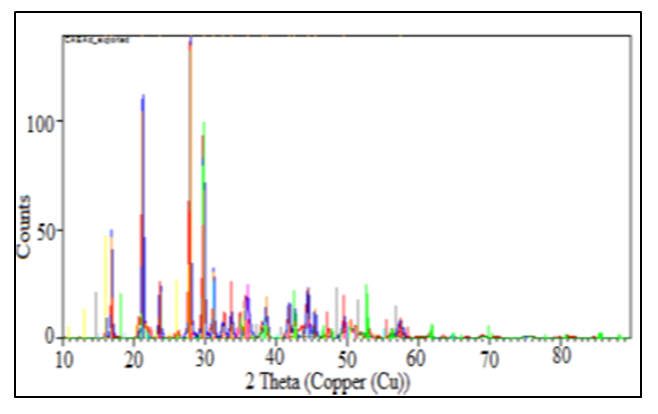 | Figure 5. Powdered diffraction pattern of HLE-2 |
3.3. Microstructural Analysis of HLE-1 and HLE-2 Hydrogels
- The microstructural analysis of the hydrogels was carried out using a scanning electron microscope. Figure 6 (A and B) shows the micrographs of both the uncross-linked and cross-linked hydrogels HLE-1 and HLE-2 respectively.
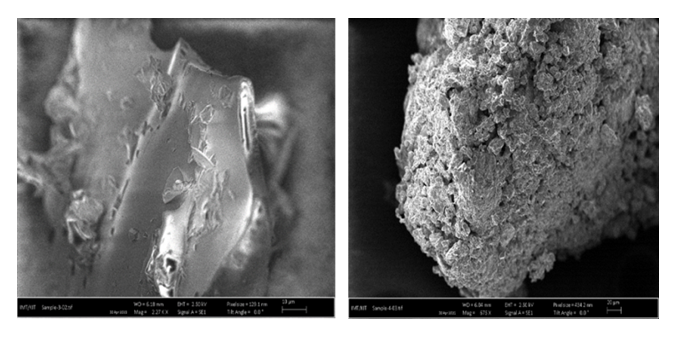 | Figure 6. SEM micrographs of the (A) HLE-1 and (B) HLE-2 hydrogel |
3.4. The Effect of Lemon Juice Dosage on the Swelling Capacity of HLE-2 Hydrogel
- Figure 7 shows the percentage swelling obtained when 2.0 g of the hydrogel was immersed in distilled water.
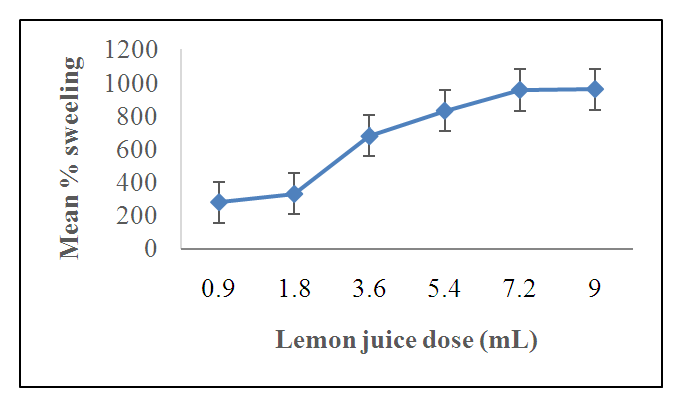 | Figure 7. The effect of lemon juice dosage on the percentage swelling of 2.0 g of HLE-2 |
3.5. The Effect of Maleic Acid Dosage on the Swelling Capacity of HLE-2 Hydrogel
- Figure 8 shows the effect of varying dosages of maleic acid cross-linker on the swelling capacity of super absorber hydrogel HLE-2.
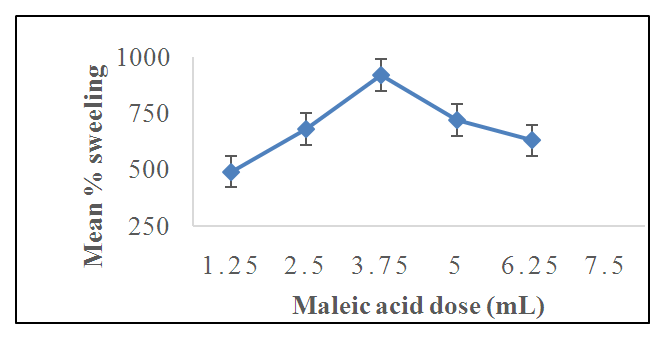 | Figure 8. The effect of maleic acid dosage on the percentage swelling of 2.0 g of HLE-2 hydrogel immersed in 500 mL of distilled water and swelling period of 24 hours |
3.6. Effect of Contact Time on the Swelling Capacity of the Hydrogel
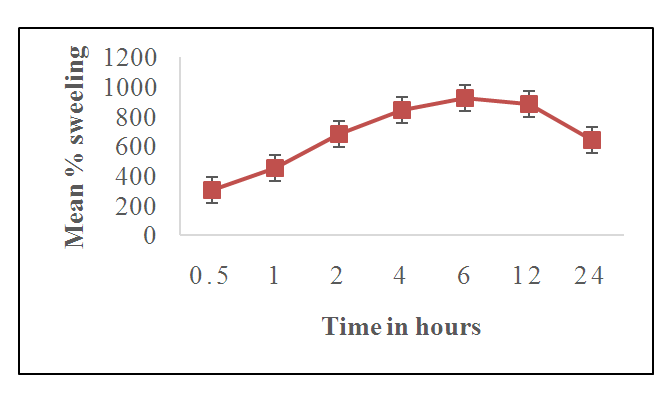
- Figure 9 shows the effect of contact time on the swelling capacity of green superabsorbent hydrogels HLE-2 at different contact times.
4. Conclusions
- The study showed that the cross-linked hydrogel prepared using LJ, EDA, and maleic acid in the volume ratio of 144:90:75 respectively produced maximum water absorption as well as the swelling capacity of 925%. The appearance of FT-IR symmetric stretching -COO- peak at 1079.83 cm-1 is an indication of successful ester linkage in the formation of HLE-2 cross-linked hydrogel. XRD analysis of the hydrogels showed that cross-linking transformed the hydrogel from amorphous to crystalline nature. SEM analysis suggested a rigid, concrete, and smooth surface in HLE-1 as compared to the uneven surface with the dispersal of a polar phase in HLE-2. HLE-2 showed a maximum swelling capacity of 925% when subjected to 6 hours in 500 mL deionized water. The hydrogel synthesized being a good water absorber has a high potential of being used in agriculture to improve on the yields especially in arid and semi-arid regions.
ACKNOWLEDGEMENTS
- The authors gratefully appreciate the insight assistance offered by the department of chemistry of Kenyatta University and the department of science technology and engineering of Kibabii University during the research period.
Declaration of Interest
- The authors declare no conflict of interest in the work described in this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML