-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2021; 11(1): 1-4
doi:10.5923/j.cmaterials.20211101.01
Received: Mar. 13, 2021; Accepted: Mar. 29, 2021; Published: Apr. 3, 2021

Composite Material Based on Clay and TiO2 for Wastewater Treatment
Andrianainarivelo Mahandrimanana1, Andriamirindramanana Herison Lunard2, Rasolomampianina Rado3
1Lecturer at the Faculty of Science of the University of Antananarivo, Department Process and Industrial Ecology
2Faculty of Science of the University of Antananarivo
3Research Director at the National Center for Environmental Research Antananarivo
Correspondence to: Andrianainarivelo Mahandrimanana, Lecturer at the Faculty of Science of the University of Antananarivo, Department Process and Industrial Ecology.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The main objective of this research is to find a system capable of treating urban wastewater, both in terms of physico-chemical and bacteriological quality. The composite material made from clay and particles of TiO2 appears to be effective in eliminating pollution from urban wastewater: case of the Marais Masay lake. Physicochemical analysis (pH, conductivity, MES, turbidity, nitrate, phosphate and chloride levels, COD, BOD) and bacteriological analysis (ASR, fecal coliforms, fecal streptococci and Escherichia Coli) have made it possible to assess the quality of treated waters. The model reduced the amount of physicochemical pollution of wastewater at the end of the treatment. The pH is between 6 and 9, the conductivity is reduced, it goes from 429.33 to 273 μS/cm, the turbidity is 17.8 NTU, the SS varies from 12.5 mg/l. Nitrite and phosphate levels are 0 mg/l. The COD is less than 50 mg/l, the BOD is less than 150 mg/l all values meet the release standard. Based on these results, 99.78% of Streptococci fecal germs, 99.64% of ASR, 97.84% fecal coliforms, 98.43% Escherichia Coli package are eliminated. Microbiological and physico-chemical reveal that the treated waters are qualitatively improved.
Keywords: Urban wastewater, Pollution, Treatment, Composite materials
Cite this paper: Andrianainarivelo Mahandrimanana, Andriamirindramanana Herison Lunard, Rasolomampianina Rado, Composite Material Based on Clay and TiO2 for Wastewater Treatment, International Journal of Composite Materials, Vol. 11 No. 1, 2021, pp. 1-4. doi: 10.5923/j.cmaterials.20211101.01.
1. Introduction
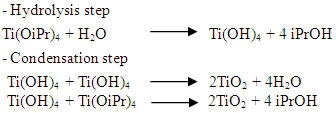
- Around two thirds of the Malagasy population do not have access to drinking water. In rural areas, the proportion is more than two out of three people. Just over half the population has access to sanitation. The situation in schools is even more critical: 79% of rural primary schools do not have water point in their enclosures, 35% do not have latrines and only about and only about 30% of the existing infrastructure the standards [1].Given the statistics, the problem of access to drinking water remains a challenge for the country. The rural population (between 75% and 80% of the Malagasy population) draws water from places which are not very clean. The source of the high prevalence of diarrhoel diseases comes from these dirty waters (well waters, river waters...) [2].There are currently different types of wastewater in Madagascar: domestic, industrial, storm and agricultural wastewater. Most of these wastewater is discharged untreated into the wild despite agricultural waste water. Most of these wastewater is discharged untreated into the wild despite their high loads of both organic and mineral pollutants.Given these circumstances, the availability of a treatment device available to everyone is necessary. Indeed, the use of local products such as clay could be beneficial for the population. Currently, there are two main types of sol-gel processes: the hydrolytic sol-gel process and the non-hydrolytic sol-gel process. Sol-gel process by mineral polymerization is one of the chemical aspects of Sol-Gel. Polymerization by the Metallic-Organic route is another aspect. Sol-Gel oxides (oxo- or hydroxo-polymers) are produced by inorganic polymerization reactions in solution from molecular precursors, usually deprotoned alcohol. Polymerization, by the hydrolytic route, takes place in two stages: hydrolysis and condensation. Hydrolysis and condensation of metallic alcohols are equivalent to nucleophilic substitution of alcoholic ligands by XOH hydroxylated species.
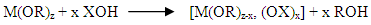 (1) Hydrolysis reaction (when X=H)
(1) Hydrolysis reaction (when X=H) It is intended to generate M-OH, reactive functions, i.e. the conversion of alcoholic functions to hydroxy functions. The solution thus obtained is called soil.(2) Condensation reaction (when X=M).It consists in the conversion of the hydroxy (or more rarely alcoholic) functions into M-O-M species. This corresponds to the formation of the mineral macromolecular network which can then be done via polycondensation reactions (formation of oxo bridges by oxolation reactions)with elimination of water or alcohol:
It is intended to generate M-OH, reactive functions, i.e. the conversion of alcoholic functions to hydroxy functions. The solution thus obtained is called soil.(2) Condensation reaction (when X=M).It consists in the conversion of the hydroxy (or more rarely alcoholic) functions into M-O-M species. This corresponds to the formation of the mineral macromolecular network which can then be done via polycondensation reactions (formation of oxo bridges by oxolation reactions)with elimination of water or alcohol: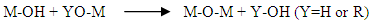 This is an oxolation. The bond between atoms is ensured by an oxo (-O-) bridge.In this study, the starting precursor is tetraisopropoxide of Titanium (Ti(OiPr)4) from Merck (bottle of 250 ml) or iPr is the isopropyl radical of semi-developed formula (CH3)2CH and deionized water. Green and white clays were used as support. Green clay is from Homeopharma (distributor of homeopathic products in Antananarivo Madagascar), the particles size is about 50μm. White clay is a pre-sifted raw material whose particles size is also about 50μm. The main objective of the study was to assess the efficiency of treatment of a clay-based and TiO2 model, with a view to eliminating pollution from urban waste water.
This is an oxolation. The bond between atoms is ensured by an oxo (-O-) bridge.In this study, the starting precursor is tetraisopropoxide of Titanium (Ti(OiPr)4) from Merck (bottle of 250 ml) or iPr is the isopropyl radical of semi-developed formula (CH3)2CH and deionized water. Green and white clays were used as support. Green clay is from Homeopharma (distributor of homeopathic products in Antananarivo Madagascar), the particles size is about 50μm. White clay is a pre-sifted raw material whose particles size is also about 50μm. The main objective of the study was to assess the efficiency of treatment of a clay-based and TiO2 model, with a view to eliminating pollution from urban waste water.2. Experimental Method
- The preparation of the composite material is divided into two steps: the first step is to synthesize titanium oxide by the hydrolytic sol-gel process, the reaction mechanism of which is summarized as follows A. Summary of titanium oxide [3,4]It is carried out in two steps:
 B. Composite material summaryClay is dried at 100°C before activating with concentrated hydrochloric acid for a few minutes.Activated clay is prepared in the form of a full disk of thickness 5mm, diameter 70 mm whose weight is about 11.33 g.0,2188g of titanium dioxide powder is weighed and dissolved completely in hydrochloric acid (30 ml).Solution is then spread over the surface of the well-prepared clay and treated at 300°C for 1 hour.C. Wastewater treatmentThe surface waste water is collected from the lake Marais Masay. The processing is done in two steps. The first step is to filter the waste water on filter paper orfine sand to remove suspended matter. The second step uses the composite material (clay- TiO2 which, placed at the bottom of the beaker and in the presence of light, [5] eliminates chemical (NO3-, Cl-) and microbiological: Anaerobic Sulfito-Reductive bacteria (ASR), Fecal Streptococci, Fecal coliforms, and Escherichia coli after 1 hour of contact. The total volume of wastewater to be treated is about 300ml.
B. Composite material summaryClay is dried at 100°C before activating with concentrated hydrochloric acid for a few minutes.Activated clay is prepared in the form of a full disk of thickness 5mm, diameter 70 mm whose weight is about 11.33 g.0,2188g of titanium dioxide powder is weighed and dissolved completely in hydrochloric acid (30 ml).Solution is then spread over the surface of the well-prepared clay and treated at 300°C for 1 hour.C. Wastewater treatmentThe surface waste water is collected from the lake Marais Masay. The processing is done in two steps. The first step is to filter the waste water on filter paper orfine sand to remove suspended matter. The second step uses the composite material (clay- TiO2 which, placed at the bottom of the beaker and in the presence of light, [5] eliminates chemical (NO3-, Cl-) and microbiological: Anaerobic Sulfito-Reductive bacteria (ASR), Fecal Streptococci, Fecal coliforms, and Escherichia coli after 1 hour of contact. The total volume of wastewater to be treated is about 300ml.3. Results and Discussions
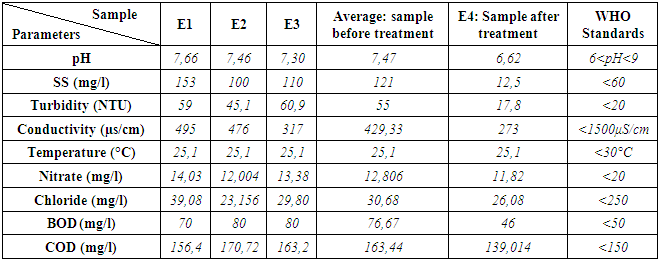
- A comparative study of the results obtained before and after treatment of wastewater is discussed in this paragraph. [6] The decree n° 2003/464 of Environmental Ministry of Madagascar inspired by WHO (World Health Organization) standards for discharge of water from a sewage treatment plant is used.(03) three wastewater samples were studied and (01) treated. Two analysis are realized for the sample E4. The average of the values obtained after two analysis are summarized in the following table:A. Physico-chemical analysis [7,8,9]The physical-chemical analysis are summarized in the following table:
|
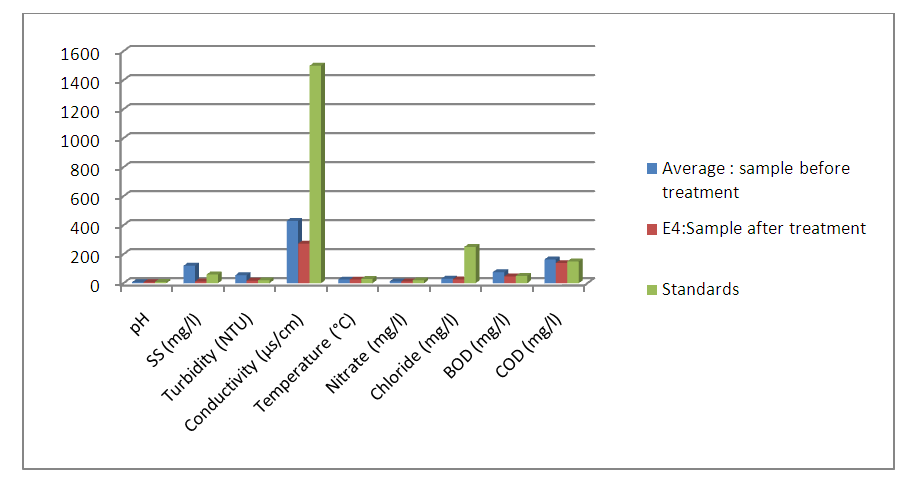 | Figure 1. Evolution of physico-chemical parameters |
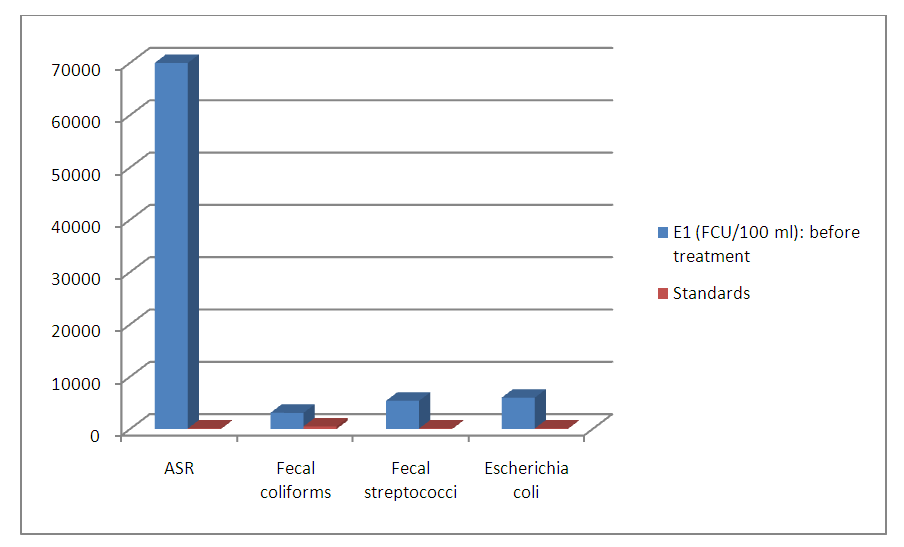 | Figure 2. Number of colonies before treatment |
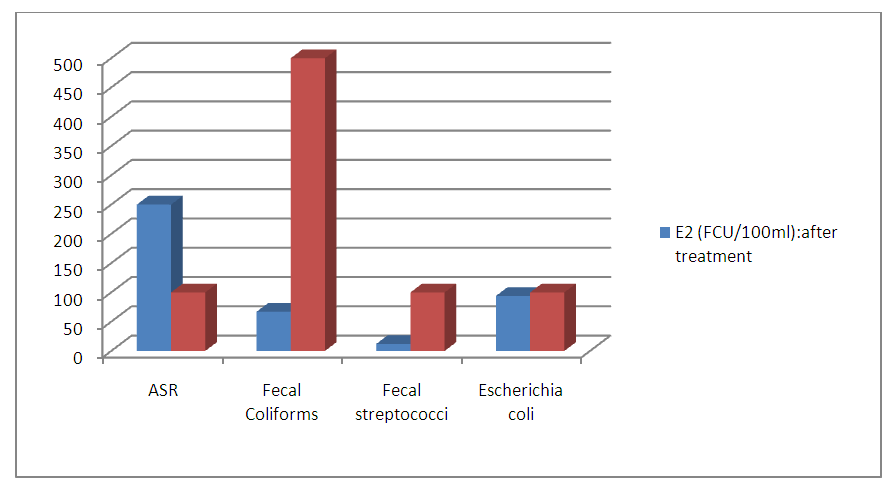 | Figure 3. Number of colonies after treatment |
4. Conclusions
- Wastewater is a particularly favorable environment for the development of all kinds of microorganisms, including pathogenic bacteria and viruses. Microbiological composition of effluents is highly conditioned by regional lifestyles and health conditions.The study is based on the reduction of the rate of harmful chemical molecules to aquatic life and the elimination of bacteria from these wastewater. It enabled us to demonstrate the effectiveness of treatment by using the composite material formed by clay and titanium dioxide nanoparticles capable of cleaning up waste water. In fact, the number of the germs decreases sharply after treatment on composite materials based on clay and TiO2. Only the ASR, resist to the treatment. The values of the physico-chemical parameters like turbidity, SS, Nitrate, chloride are below standards.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML