-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2020; 10(2): 29-36
doi:10.5923/j.cmaterials.20201002.01

Eco-Friendly Urea-Formaldehyde Composites Based on Corn Husk Cellulose Fiber
Shanta Pokhrel1, Muna Shrestha1, Miroslav Slouf2, Jakub Sirc2, Rameshwar Adhikari3
1Department of Chemistry, Tri-Chandra Multiple Campus, Tribhuvan University, Kathmandu, Nepal
2Institute of Macromolecular Chemistry, Czech Academy of Sciences, Czech Republic
3Research Centre for Applied Science and Technology, Tribhuvan University, Kathmandu, Nepal
Correspondence to: Shanta Pokhrel, Department of Chemistry, Tri-Chandra Multiple Campus, Tribhuvan University, Kathmandu, Nepal.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Cellulose fibers were extracted from dried raw corn (Zea mays) husk, is the second most widely traded cereal after wheat, by alkaline treatment (mercerization), followed by neutralization with acid; product of which underwent bleaching to produce pure form of cellulose. Urea-formaldehyde (UF) resin was prepared from formaldehyde and urea and its composite with the extracted cellulose fibers was prepared via solution casting method. Characterization of prepared samples was carried out by X-Ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM), compressive strength test and water absorption test. From the results of XRD, FTIR and SEM, extraction of pure cellulose was affirmed, crystalline nature of cellulose was confirmed and crystallite size of thus obtained cellulose was also determined by XRD. From the results of FTIR, compressive strength test and water absorption test, it was concluded that addition of cellulose fiber to UF resin increases the compressive strength and water absorption of the composite which ultimately makes the composite more bio-degradable and eco-friendly.
Keywords: Cellulose, FTIR, SEM, Urea-formaldehyde resin, Water absorption
Cite this paper: Shanta Pokhrel, Muna Shrestha, Miroslav Slouf, Jakub Sirc, Rameshwar Adhikari, Eco-Friendly Urea-Formaldehyde Composites Based on Corn Husk Cellulose Fiber, International Journal of Composite Materials, Vol. 10 No. 2, 2020, pp. 29-36. doi: 10.5923/j.cmaterials.20201002.01.
Article Outline
1. Introduction
- Bio-based industrial residues are increasing every day. Alongside is increasing cost of their management. That is why, use of bio-based industrial residue as industrial raw material is now a growing trend among entrepreneurs [1]. Value added utilization of such residues in accordance with environmental requirements has motivated many young researchers [2,3]. Every responsible proficient global citizen is now concerned about developing sustainable and renewable polymeric material which would be able to replace petrochemical based pollution- prolonging plastic materials. Natural fiber reinforced plastics prepared using biodegradable polymer as matrix are the most environmental friendly materials [4]. One of the many efforts include, reinforcing thermosetting polymers with natural fibers for making it more bio-degradable and eco-friendly. Natural fibers when used in reinforcement of polymeric materials give enhanced mechanical and thermal stability proving them to be a better option of pure petrochemical based polymeric materials [5]. Cellulose, the most abundant biopolymer on earth, is chemically defined as a linear homopolymer composed of β-1, 4-linked glucose molecules, [6,7]. It provides excellent properties such as high mechanical properties, high strength, low thermal expansion, low density and biodegradability [6,8]. The recent demand in materials research is to develop materials which comprise excellent features such as enhanced mechanical properties and thermal stability, biodegradability, being eco-friendly, and low-cost [6]. Therefore, cellulose fibers are found to be one of those interesting co-product of bio-based industries which can be used to fill or reinforce thermosetting polymers for preparation of natural fiber composites. These materials facilitate waste management by biological processes having various applications in the economic fields where the biodegradability and renewability are preferred characteristics features [9]. Considerable contribution has been given in research using thermosets and thermoplastics as matrixes and natural fibers as reinforcements for the preparation of composites. The most commonly used thermosets are phenol formaldehyde, resorcinol formaldehyde, epoxy and urea–formaldehyde resins [10,11]. Urea-formaldehyde (UF) resin, one of the most important formaldehyde resin adhesives, is a polymeric condensation product of formaldehyde with urea [12]. However, easily available cellulose fiber used in paper and textile industries is not yet investigated as reinforcements, despite its high potential of replacing traditional glass and carbon fibers. Natural fibers are easily available, light weight, cheaper, easily separable, non-corrosive and biodegradable. Additionally, they have good thermal properties in comparison to traditional reinforcement materials such as glass fiber, carbon fiber etc. [5]. Keeping in mind these massive advantages of cellulose fibres, the present work is designed to fabricate corn husk (Zea mays) fiber as reinforced urea-formaldehyde based polymer composites and study the mechanical properties and biodegradability via compressive test and water absorption test respectively.
2. Materials and Methodology
2.1. Materials Required
- Sodium hydroxide (97% Merk); Acetic acid (99.5% Fisher Scientific); Hydrogen peroxide (30%); Urea (99.5%); Formaldehyde (37% Fisher Scientific) and Sulphuric acid (97% Merk); were of analytical grade and used without further purification. Corn husk was obtained from local farm house, Panchkhal, Kavrepalanchowk District, Nepal.
2.2. Extraction of Cellulose From Corn Husk by Chemical Modification
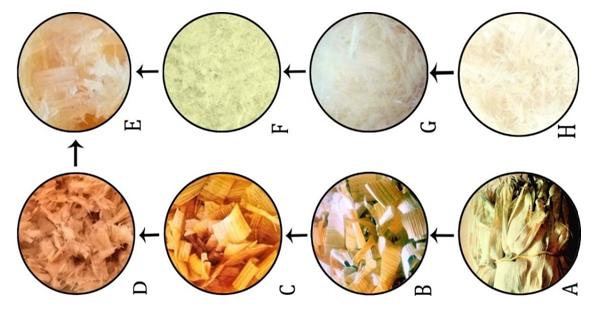
- Different stages involved in the extraction of cellulose from corn husk are depicted in Figure 1. According to Chirayil et al., pre-treatment with sodium hydroxide and sodium chlorite (bleaching), has removed the noncellulosic constituents resulting in fibers with high cellulose content [13]. Therefore, three simple steps of mercerization (alkali treatment), neutralization and bleaching were involved to obtain high cellulose content material from corn husk. I. Mercerization; Corn (Zea mays) husk were sun dried until completely dehydrated and cut into small pieces of almost 2 to 3 cm and treated with 0.5 N sodium hydroxide (NaOH) solution for 3 hours at 70°C with 5% weight of cornhusk in alkali solution. This process removes lignin, hemicellulose, waxes and other surface impurities of fiber. Therefore this process is also called mercerization [1].
2.3. Synthesis of Urea Formaldehyde (UF) Resin
- The UF resin was synthesized in laboratory (Scheme 1) by following standard protocols [16,17] with slight modification; using 1:3 (W/V) ratio of urea and formaldehyde. Formaldehyde (18 mL) in the beaker was heated on paraffin oil bath for 10 min and 6 g of urea was added to it (1 gram urea at a 1 min interval). This procedure was carried out in a magnetic stirrer for consistency in the stirring procedure. Few drops of concentrated sulphuric acid was added to the UF resin thus formed and further heated until viscous mass was obtained. Thus, obtained UF resin viscous mass was poured into plastic ice tray with grooves of dimensions (50 cm ×30 cm ×20 cm) and was pre cured a 50°C for 24 hours and post cured at 80°C for 48 hr.
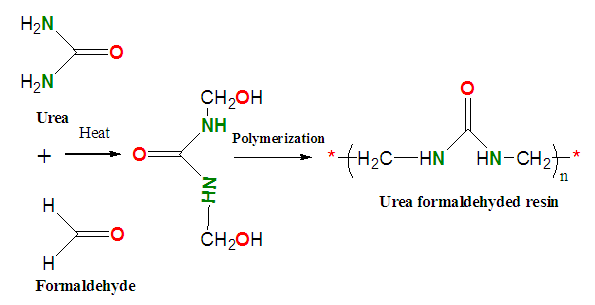 | Scheme 1. Schematic route for the synthesis of urea-formaldehyde (UF) resin |
2.4. Preparation of Urea Formaldehyde/Corn Cellulose Fibers (UF/CC) Composites
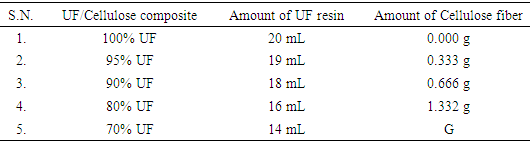
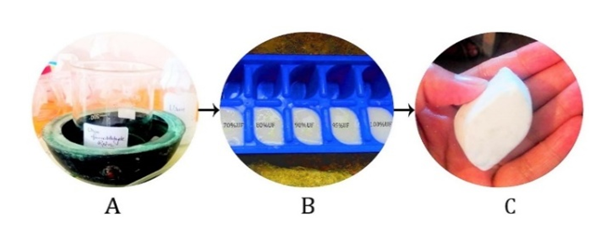
- Cellulose fibers based urea formaldehyde composites were prepared using solution casting method (Figure 2). The composites of different compositions 0/100, 5/95, 10/90, 20/80 and 30/70 (by weight) of fiber were prepared (Table 1). Thus obtained natural fiber cellulose was characterized by XRD, FTIR and SEM and its composites by FTIR, compressive strength test and water absorption test.
|
2.5. Characterization Techniques
2.5.1. Fourier Transform Infrared Spectroscopy (FTIR)
- The FTIR analysis was carried out in Central Department of Chemistry, Tribhuvan University, Kathmandu, Nepal by using IR Prestige-21 Spectrometer Shimadzu, Singapore. The spectra were collected in the spectral range of 4000-400 cm-1 with spectral resolution of 4 cm-1 in ATR mode.
2.5.2. X-Ray Diffraction (XRD)
- XRD (Bruker D2 Phase) with a monochromatic CuKα radiation source (λ=0.15406 mm) at scanning rate of 0.1 degree per step and 2θ ranging from 20 to 80 degree at Nepal academy of Science and Technology (NAST), Khumaltar, Lalitpur, Nepal. The accelerating voltage 35 KV and emission current of 30 mA were used.The average crystallite size of the extracted cellulose was determined with the help of Debye Scherer Formula [18] (equation 1):
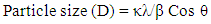 | (1) |
2.5.3. Scanning Electron Microscopy (SEM)
- Extracted cellulose fibers were characterized by SEM (FEGSEM MAIA3, HV=2kV, model 2016, Tescan, Czech Republic) to have better understanding of the surface structures and morphologies.
2.5.4. Compressive Strength Test of UF/CC Composites
- The UF/CC composites were analyzed by measuring the compressive strength which was performed according to directives of American Society for testing and material (ASTM) for each sample. It was carried out by Compressive Testing Machine (CTM, capacity 500 kN, Harris & Tarris Co.) at the Central Material Testing Laboratory at Pulchowk Engineering Campus, Pulchowk, Lalitpur, Nepal. The calibration factor of the instrument was 3.7 Kg and blocks of average 15 g were tested.
2.5.5. Water Absorption Test of UF/CC Composites
- Water absorption test is required to predict the biodegradability of the composites. Water absorption on the materials allows microorganisms, such as bacteria and fungi to grow and utilize cellulose as a carbon source. The results implied that the sample with higher cellulose contents exhibited better biodegradability due to the fact that biodegradation is naturally caused by the penetration of the microorganisms using water as a medium. [19,20]. Composites with same dimension were immersed in clean distill water. Weight measurements were taken at of 6 hr, 24 hr, 48 hr, 72 hr, and 144 hr of immersion initially and weekly thereafter. The amount of absorbed water was calculated using the following equation 2 [21,22]:
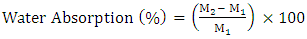 | (2) |
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopy (FTIR) of Corn Husk and Extracted Cellulose
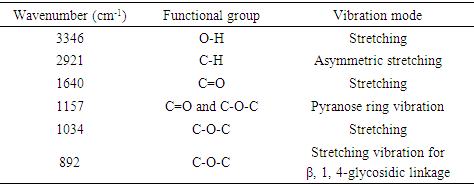
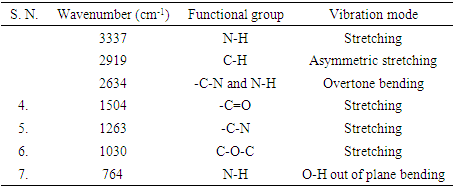
- Figure 3 displays the FT-IR vibrational spectrum of wave number 4000–500 cm-1 of natural fiber and cellulose. It showed broad absorption bands between 3600-3000 cm-1 which represents the O-H stretching vibration and very strong intra and intermolecular hydrogen bonds vibration of O-H of methyl and methylene of cellulose which is more prominent in case of extracted cellulose in comparison to the raw corn husk [23]. An axial C-H stretching vibration can be seen at 2921 cm-1 which is also more prominent in case of cellulose. FTIR peaks at 2915-2820 cm-1 are by C-H stretching. The peak at 1640 cm-1 is representing C=O stretching of hydrocarbon [23,24]. Characteristic stretching of C-O and C-O-C pyranose ring vibration of cellulose is represented by 1157 cm-1 peak [4,25]. The strong absorption band at 1034 cm-1 is observed which is characteristic of cellulose as supported by Fan et al. [15].
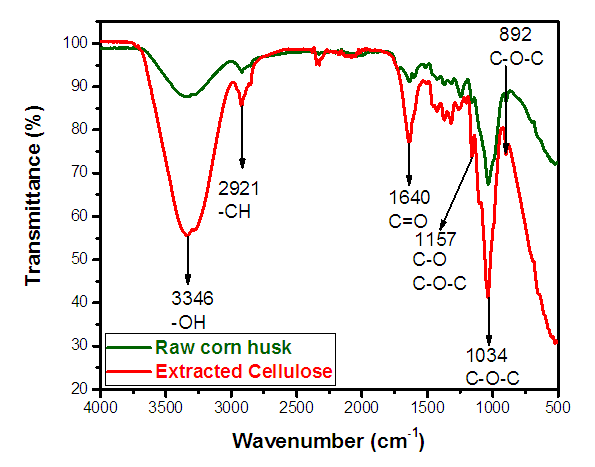 | Figure 3. Fourier Transform Infrared Spectroscopy of cellulose and corn husk |
|
3.2. X-ray Diffraction (XRD) Analysis of Corn Husk and Extracted Cellulose
- Figure 4 compares the XRD patterns of raw corn husk and extracted cellulose. The average crystalline size of the extracted cellulose was determined 70 nm with the help of Debye Scherer Formula [18] (equation 1). XRD analysis of cellulose revealed the signatures characteristic of cellulose I structure, with XRD peaks located at 22.4 and 34.6° 2θ which correspond to (021) and (040) latiice plane respectively [30]. Whereas weak broad peak at 28.0° 2θ corresponds to a combination of 130, 131, 221, 227, 230 and 310 reflections [31]. The peak at 28 is very broad and weak which is affected by scattering from the paracrystalline structure and by moisture [31]. Conversely, the XRD pattern of raw corn husk appeared to be amorphous, with slight crystallinity observed at 22.0° 2θ that correspond to (021) crystallographic planes of cellulose II. On removing non-cellulosic constituents of corn husk by chemical modification, the intensity of peak become more intense and defined. Crystallinity of raw fiber is characteristically lower in comparison to that of cellulose which proves that chemical treatment successfully increased the cellulose fiber crystallinity due to removal of hemicellulose and lignin contents during the chemical treatment as also reported by Janoobi et al. [32].
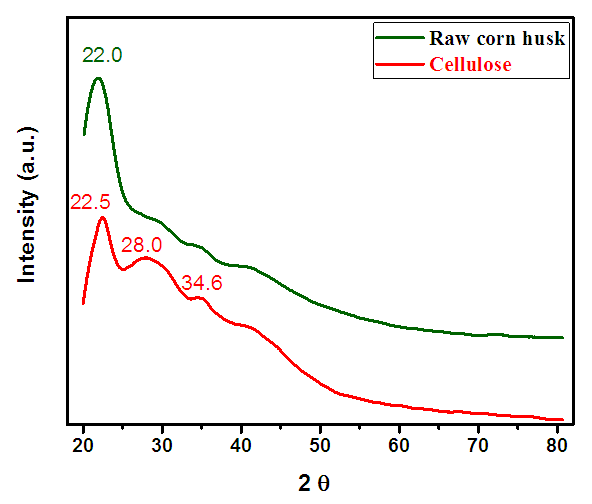 | Figure 4. X-ray Powder Diffraction pattern of cellulose and corn husk |
3.3. Scanning Electron Microscopy (SEM)
- The surface morphology of the cellulose fibers was examined by scanning electron microscopy which, selected SEM micrographs are shown in Figure 5. The micrographs showed vascular texture of fibrils. The SEM results suggested that mercerization caused fibrillation and breaking of fibers to small pieces that increased surface area as previously studied by Kunusa et al in corncobs [33]. Petersson and Oksman [34] studied extensively microcrystalline cellulose (MCC) as reinforcement for polymers. MCC, particles of hydrolyzed cellulose consist very large amount of cellulose microcrystals together with amorphous areas. Exfoliation can be improved by swelled MCC prior to solution casting processing [34].
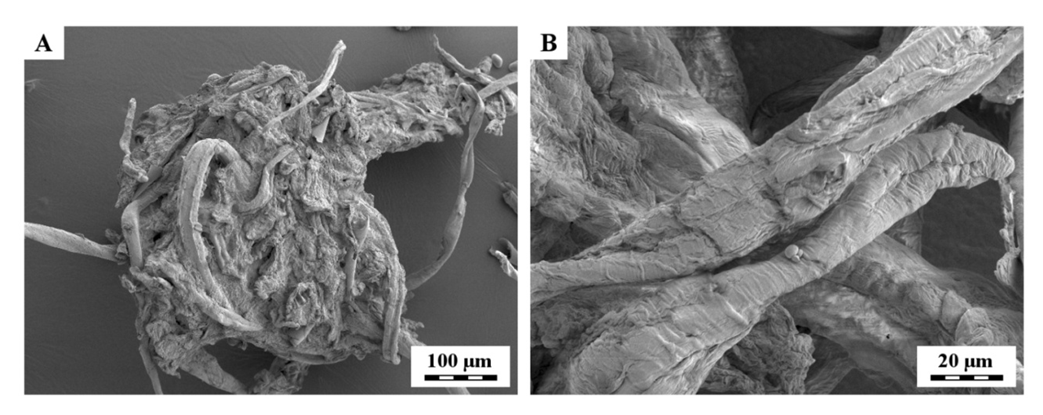 | Figure 5. Scanning electron micrograph of dried cellulose fibers at (A) lower and (B) higher magnification |
3.4. Characterization of Urea Formaldehyde/Corn Husk Cellulose Composites
- Characterization of urea formaldehyde/cellulose composite was carried out by FTIR, compressive strength test and water absorption test to find the mechanical strength and biodegradability or eco-friendly nature of the matrix composite.
3.4.1. Fourier Transform Infrared (FTIR) Spectra of Urea Formaldehyde/Corn Husk Cellulose (UF/CC) Composite
- Figure 6 present the FT-IR vibrational spectrum of wave number 4000-500 cm-1 of Urea formaldehyde/cellulose composite. Urea formaldehyde resin showed characteristic peaks at 3290 cm-1 for N-H stretching of primary amine urea, 2962 cm-1 for C-H stretching peak of UF, 1650 cm-1 for C=O stretching vibration and 771 cm-1 for N-H bending of secondary aliphatic amines [16,17]. The distinct absorption peak at 3337 cm-1 of urea formaldehyde resin is attributed to N-H stretching of primary aliphatic amines. The peaks at 3335-3340 cm-1 attributes O-H stretching intramolecular hydrogen bonds. FTIR peaks at 2915-2820 cm-1 are due to C-H stretching [16,17].
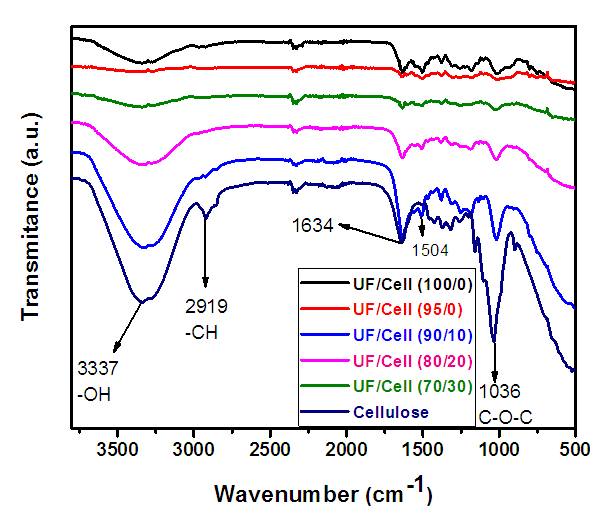 | Figure 6. Fourier Transform Infrared spectra of urea formaldehyde/corn husk cellulose composites |
3.4.2. Compressive Strength Test
- From the graph (Figure 7), it is seen that increase in amount of cellulose in UF resin composite increased the compressive strength which supports the work by Sharma et al for other similar UF resin reinforced with natural fiber [39] and Singha and Thakur for UF resin itself [40,41]. This suggests that UF resin reinforced by cellulose fibers is capable enough to to acquire good mechanical properties. The decrease in hardness in the fiber composite from pure composites attributes to fiber-matrix interfacial bond formed in the composites. This is found to improve the mechanical properties of fiber composites by making it more flexible and shock resistant [39].
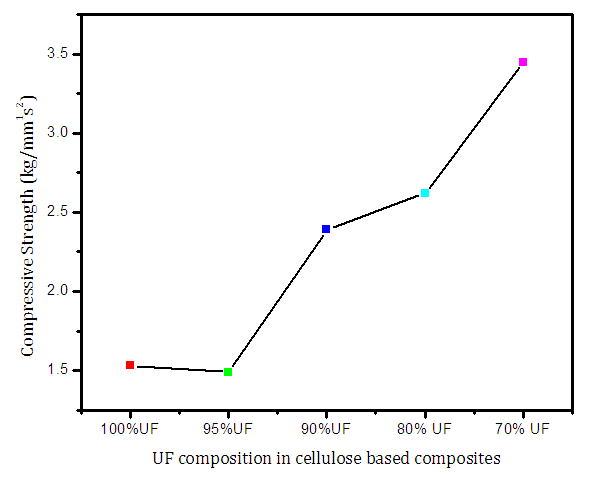 | Figure 7. Compressive strength of urea formaldehyde/corn husk cellulose composites |
3.4.3. Water Absorption Test
- The samples were found to absorb water significantly on initial week and the increment of absorbing percentage became almost constant thereafter.
|
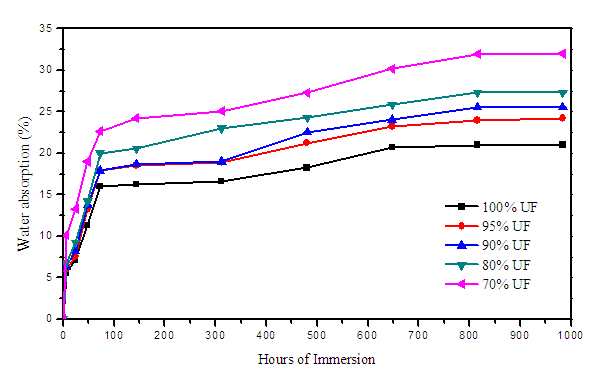 | Figure 8. Water absorption of Urea-Formaldehyde/corn husk cellulose |
4. Conclusions
- Cellulose fibers were successfully extracted from corn husk by chemical modification using mercerization which was confirmed by XRD and FTIR results. Urea-formaldehyde matrix was reinforced with cellulose fiber obtained from corn husk with different composition of composites were prepared via solution casting method. The FTIR spectra showed corresponding peak of cellulose and UF resin suggesting well distribution of fiber in polymer. Some shifts in peaks of FTIR suggested feeble chemical interaction between matrix and reinforcement. Prepared composites were analyzed for mechanical property (compressive strength) and it was found that fiber loading increased the mechanical strength of composites upto 30% of fiber loading. Additionally, water absorption test concluded that water uptake of composites increases with increased loading of cellulose which ultimately makes the composite more bio-degradable and eco-friendly.
ACKNOWLEDGEMENTS
- MS is grateful towards Tri-Chandra Multiple Campus, Tribhuvan University, Kathmandu, Nepal for providing the research opportunity and thanks material testing lab of Pulchowk Engineering College, Lalitpur, Nepal for assisting with compressive strength test, Nepal Academy of Science And Technology (NAST), Lalitpur, Nepal for helping with XRD results, Central Department of Chemistry, Tribhuvan University, Kathmandu, Nepal for supporting with FTIR results.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML