-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2020; 10(1): 1-9
doi:10.5923/j.cmaterials.20201001.01

Moisture Effect on Properties of Out-of-Autoclave Laminates with Different Void Content
Afshin Bayatpour , Mehdi Hojjati
Concordia Center for Composites, Concordia University, Montreal, Quebec, Canada
Correspondence to: Mehdi Hojjati , Concordia Center for Composites, Concordia University, Montreal, Quebec, Canada.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Fabrication of large structures using out-of-autoclave prepreg materials will lead to a great amount of savings in manufacturing costs. In the out-of-autoclave processing method, the presence of voids inside the laminate has been an issue due to the lack of high pressure during manufacturing. This study aims primarily to observe the moisture absorption response of composite samples containing different levels of void. By changing the vacuum level inside the bag during the manufacturing process, three different unidirectional laminates at three levels of void have been manufactured. After immersing the samples in warm water at 60°C for about one year, the moisture absorption level was monitored and then diffusion coefficients were calculated using Fick’s law. Results show that the moisture absorption coefficient changes by %8 within the experimental range of void contents. The mechanical behaviour of these laminates has been studied at four different moisture levels by performing dynamic mechanical analysis (DMA) and short beam shear tests. Empirical results indicate that, in general, interlaminar shear strength and glass transition temperature decrease by moisture build-up inside the samples. DiBenedetto equation is proposed to make a correlation between the moisture content and glass transition temperature.
Keywords: Out-of-autoclave materials, Moisture absorption, Moisture diffusivity
Cite this paper: Afshin Bayatpour , Mehdi Hojjati , Moisture Effect on Properties of Out-of-Autoclave Laminates with Different Void Content, International Journal of Composite Materials, Vol. 10 No. 1, 2020, pp. 1-9. doi: 10.5923/j.cmaterials.20201001.01.
Article Outline
1. Introduction
- Polymer matrix composites, especially carbon fiber reinforced polymers (CFRP) have become attractive in the last decades in various industries due to their excellent mechanical properties to density ratio. In some of the applications including aerospace, civil infrastructure and marine sectors, these materials are subjected to harsh environments such as water and high humid conditions. Mechanical properties of composite materials are very sensitive to the different environmental conditions such as temperature, humidity, and corrosive environments. These environmental conditions can affect the matrix and matrix-fiber interface properties. To estimate the durability of the material exposed to such environment, it is necessary to study the mechanical properties of them in harsh environments under different loading conditions. To study the effect of moisture on composite materials, it is essential to have a look at the chemical reactions between the material and water molecules to know how moisture diffuses through the laminate. Due to the fact that the moisture cannot penetrate through the fibers, resin properties become very critical and will be affected by the moisture diffusion [1-2]. There are two factors which affect the diffusion of penetrant molecules into polymeric material: a) availability of molecular size holes between polymer chains and b) bonding forces between penetrant molecules and polymer [3-4]. Availability of molecular size holes depends on various resin characteristics such as polymer microstructure, crosslink density, stoichiometry, and cohesive energy density while the chemical nature of penetrant molecules and polymer determine the essential bonding force between them. As the hydrogen bonding sites in polymer chains increase, the attraction between water molecules and polymer increases [5-6]. Moisture diffusion into the polymer can be controlled by many factors. Movement of a penetrant through the polymer can be different in each polymer because the nature of polymer plays a critical role in moisture transport. The amount of free volume and segmental mobility in the polymer are the factors which affect the diffusion directly. There are some other factors like degree of unsaturation, degree of crosslinking and degree of crystallinity which can affect diffusion indirectly by changing the segment mobility. Moreover, glass transition temperature has a significant influence on moisture diffusion. As Tg reduces in polymers, segment mobility and moisture diffusivity increase. Furthermore as molecular size of polymers increases, segment mobility of the molecular chains decreases drastically which leads to lower diffusion rate and moisture content [1,7]. In the case of similarity in the nature of polymers and the crosslink density, the nature of the crosslink can affect the diffusion procedure. It is shown that as molecular chain flexibility increases, the maximum level of moisture uptake and diffusion rate increase [7]. Increasing the plasticisers in polymer chains can enhance the rubbery properties and increase the segment mobility of polymer, which can lead to a jump in diffusion coefficient and reduces the solubility of the polymer [7]. Molecular shape and size is another factor that can affect the rate of diffusion in the polymeric matrix. Several studies thus far have linked the size of penetrant with diffusivity in polymers. It has been reported that as the size of penetrant molecules increases, the rate of diffusion decreases [8,9]. The nature of reinforcement, its compatibility with the polymer matrix and also its adhesion properties are the dominant factors in diffusion of a penetrant in filled polymers [7]. When a filler is not compatible with the matrix nature, the amount of void content increases at the interface, which can increase the free volume and also the rate of diffusion. On the other hand, in the case of compatibility of the reinforcement and the polymer system, the filler can go through the void contents and reduce the diffusivity [10]. Moreover, using the fillers can reduce and restrict the mobility of the penetrant, which can again lead to reduction in diffusivity [11-12]. The difference in the coefficient of thermal expansion (CTE) and the coefficient of moisture expansion (CME) between the fibers and the matrix can make the swelling an anisotropic phenomena in composite materials. Swelling decreases by increasing the fiber volume fraction and can be negligible in the presence of a strong interface [13-14].In most cases, increase in temperature leads to decrease in solubility and increase in both diffusivity and maximum moisture content in the composite materials [15]. Gillat et al, studied the moisture diffusion of T300 graphite/epoxy under different external loadings at 25°C, 60°C and 80°C for two different cross-ply laminates. They observed an increase in diffusivity and maximum moisture content as immersion temperature increased [16]. Lv et al, discovered that an increase in diffusivity at a higher immersion temperature is due to an increase in molecular chain relaxation and reduction in Tg and molecular bonding strength at higher temperatures [17].Composite structures are often cured in an autoclave to achieve the required aerospace grade quality. However, curing large structures requires access to large autoclaves which is limited and expensive. As a result, fabrication of large structures using out-of-autoclave prepreg materials will lead to a great amount of savings in manufacturing costs. In the out-of-autoclave processing method, the presence of voids inside the laminate has been an issue due to the lack of high pressure during manufacturing. This research aims to study the effects of moisture on properties of an out-of-autoclave unidirectional carbon fiber composite material. The main focus is to investigate the moisture absorption behavior (moisture diffusivity) and mechanical properties of the laminates at different levels of void contents. Laminates are made with three different void contents and then subjected to the moist environment for one year. At different moisture level, samples were weighted and then mechanically tested. Effect of moisture on diffusivity coefficient, glass transition temperature, and interlaminar shear strength will be investigated.
1.1. Fick’s Law for Moisture Absorption and Desorption
- Fickian diffusion is the most common diffusion model to describe the moisture absorption and desorption through polymeric material. This model was described by Adolf Fick in 1855 for steady state (Fick’s first law) and non-steady state (Fick’s second law) diffusions [18]. The driving force which leads to the molecular transportation is difference in concentration, which moves the penetrant molecules from higher concentration areas to lower concentration areas in the solvent. This movement ends when the polymer reaches the equilibrium mass concentration. Fick’s second law explains the moisture diffusion when concentration gradient changes as time goes on. The following equation is the one-dimensional Fick’s second law of diffusion equation with the constant diffusivity assumption [19,20]:
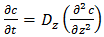 | (1) |
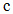 is the moisture concentration which is the absolute amount of absorbed moisture in a material and expressed as the mass of moisture per unit volume. z is the coordinate through-the-thickness and t is time.
is the moisture concentration which is the absolute amount of absorbed moisture in a material and expressed as the mass of moisture per unit volume. z is the coordinate through-the-thickness and t is time. 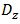 is the laminate through-the-thickness moisture coefficient of diffusion or moisture diffusivity coefficient. For an infinite plate (width and length
is the laminate through-the-thickness moisture coefficient of diffusion or moisture diffusivity coefficient. For an infinite plate (width and length  thickness), Jost derived the following solution [19]:
thickness), Jost derived the following solution [19]: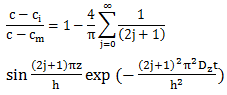 | (2) |
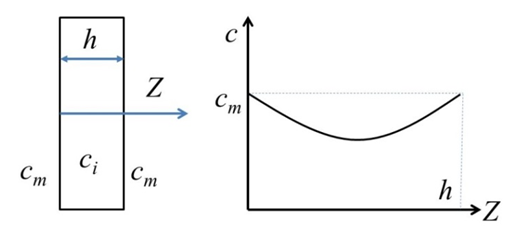 | Figure 1. Typical through-the-thickness moisture concentration at time t |
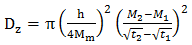 | (3) |
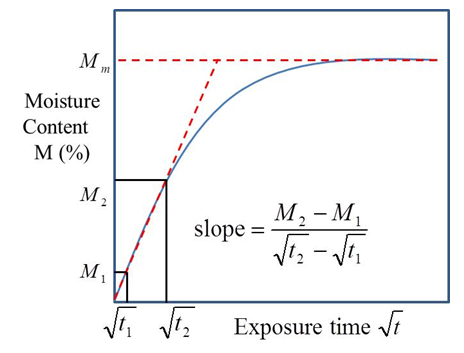 | Figure 2. Moisture absorption process based on Fick’s law |
2. Materials and Manufacturing
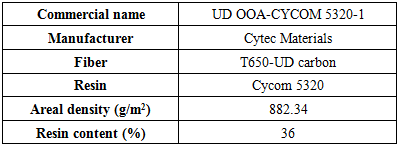
- Material used to manufacture samples is CYCOM 5320-1 which is produced by Cytec Engineered Materials Inc. This is one of the most common out-of-autoclave materials used in aerospace applications. Material specification is given in Table 1.
|
|
|
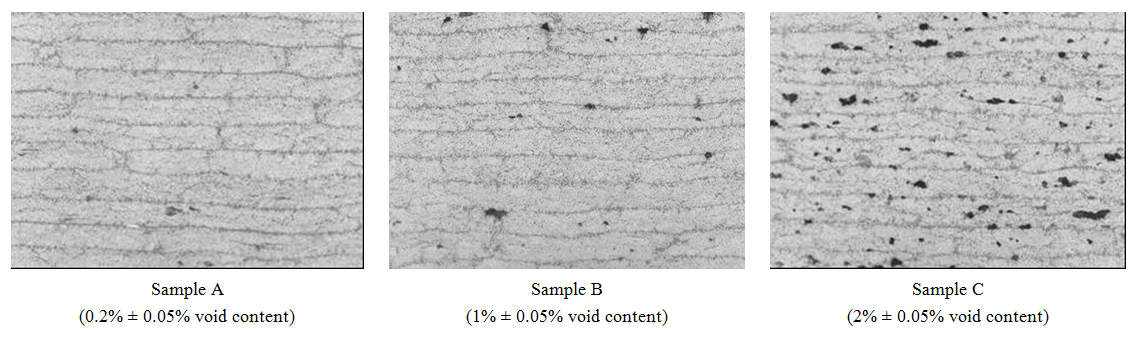 | Figure 3. Cross sections of three different samples |
3. Experiment
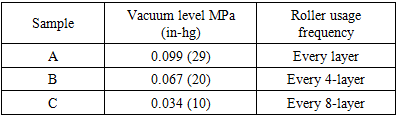
- After cutting the samples, it is crucial to dry the samples. After measuring the initial weight of the samples, the samples were put in an oven for different times at 60°C. During the drying procedure, the weights of the samples were monitored and the procedure was stopped when the weight does not change more than 0.02% as suggested by ASTM standard. At this step, the last monitored weight was labeled as Wd. Since our objective is to obtain the through the thickness diffusivity and moisture absorption behaviour of composite laminates, the samples were bonded by stainless steel foil at the edges (Figure 4). This prevents the penetration of the moisture from the edges and the only passage for moisture to get into the laminate will be from the laminate surface. Before starting the moisturising, the weights of the samples were measured to consider the extra weight the steel foil. This weight is called WAl. A slow cooker pot was used for moisturizing the samples. The pot was filled with the water and the samples were placed inside the pot and temperature was set to 60°C [20,24]. Temperature was monitored through an independent thermocouple which was placed inside the laminate during manufacturing. Every week the samples were removed, cleaned and weighted (Wi) and then put back in the pot.
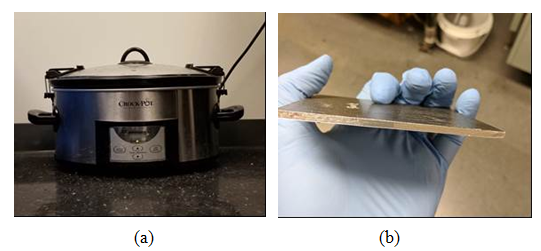 | Figure 4. a) Moisturising pot, b) Sample after adding aluminum foil to the edges |
4. Results and Discussion
4.1. Moisture Diffusivity Coefficient
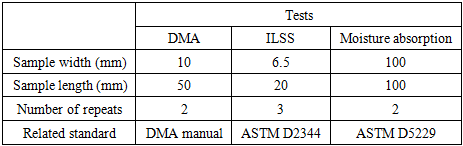
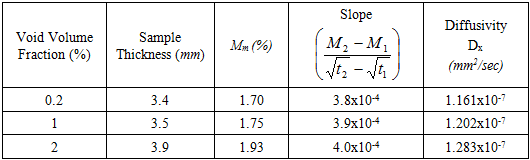
- The procedure proposed by ASTM was followed to run the moisture tests and to collect and analyze the data [20]. The sample weights of six unidirectional carbon/epoxy laminates at three different void contents were measured and recorded during different intervals within one year exposure time. The provided results are average of weight measurement of two specimens. The moisture content was calculated from change of weight using the following equation [20]:
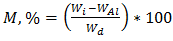 | (4) |
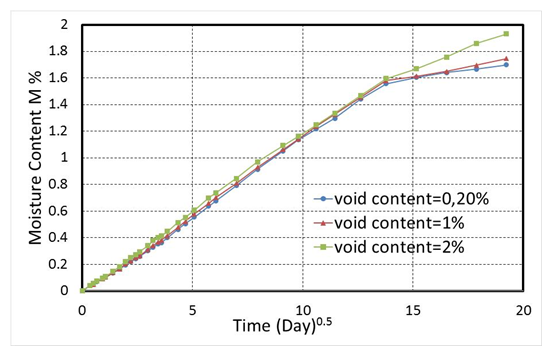 | Figure 5. Experimental data from moisture absorption process of composite laminates at three different levels of void |
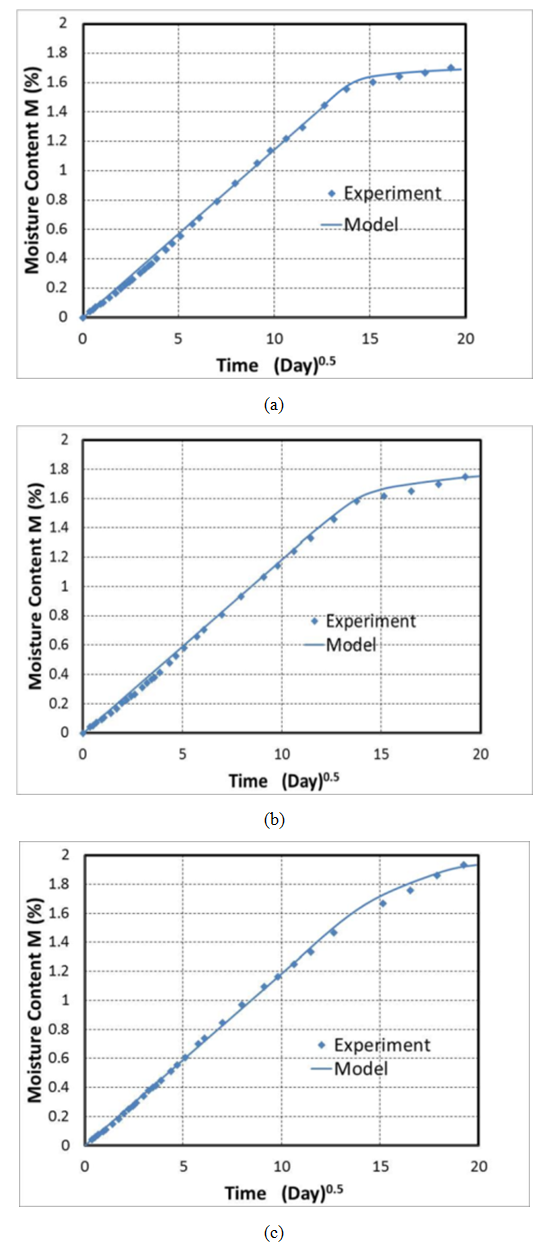 | Figure 6. Moisture content vs time for three different void contents a) 0.2%, b) 1%, and c) 2% |
|
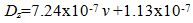 | (5) |
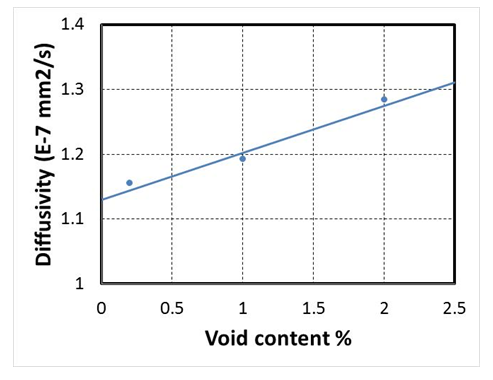 | Figure 7. Variation of moisture diffusivity and void content |
4.2. Interlaminar Shear Strength
- Short beam shear test was performed to measure interlaminar shear strength. The load-displacement curve for a dry sample with the minimum void content (0.2%) is shown in Figure 8a. All the samples were examined for the mode of failure based on the ASTM D 2344 standard. Typical sample failure is layer slippage at the layer boundaries as shown in Figure 8b.
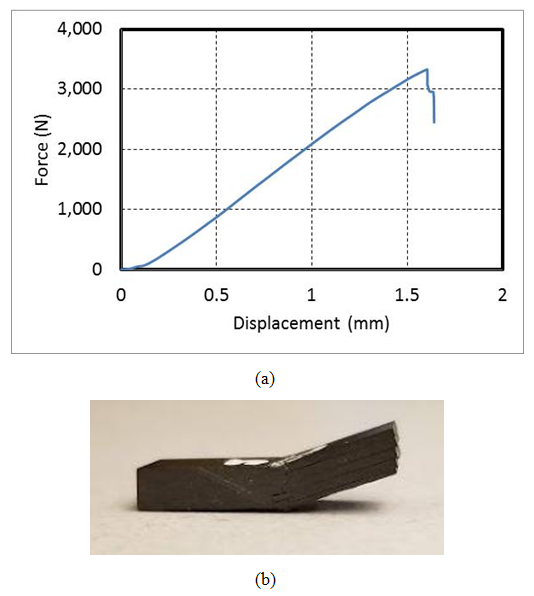 | Figure 8. a) Force-displacement curve for a short beam shear test, b) Mode of failure |
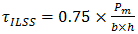 | (6) |
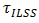 is the interlaminar shear strength (MPa),
is the interlaminar shear strength (MPa), 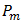 is the maximum load observed during the test (N), b is the measured laminate width (mm) and h is the average laminate thickness (mm). Figure 9 shows how ILSS varies for the composite laminate after exposure of samples to the moisture environment. Results are the average of three (3) specimens. Comparing the ILSS values for two panels clearly indicates the effect of the void on the composite properties. For the dry samples, ILSS reduces by almost %15 from 103 MPa to 87.6 MPa. Similar trend can be seen on the other moisture levels.
is the maximum load observed during the test (N), b is the measured laminate width (mm) and h is the average laminate thickness (mm). Figure 9 shows how ILSS varies for the composite laminate after exposure of samples to the moisture environment. Results are the average of three (3) specimens. Comparing the ILSS values for two panels clearly indicates the effect of the void on the composite properties. For the dry samples, ILSS reduces by almost %15 from 103 MPa to 87.6 MPa. Similar trend can be seen on the other moisture levels.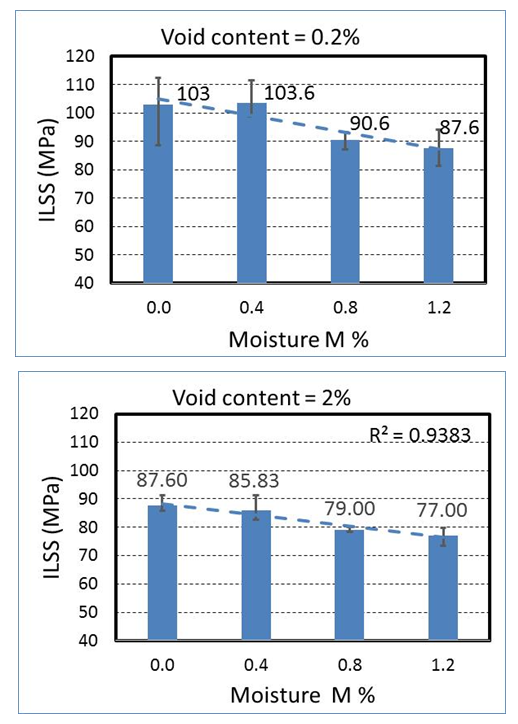 | Figure 9. Interlaminar shear strength of unidirectional laminates containing 0.2% and 2% void contents at four different moisture levels |
4.3. Glass Transition Temperature
- Glass transition temperature is another valuable output from DMA tests. Tg is the temperature which the material goes from glassy state to the rubbery state with a sharp drop in stiffness. The DMA results for sample with minimum void content at dry condition are shown in Figure 10. As temperature increases, the storage modulus of the sample decreases while loss modulus of the sample increases which can cause an increase in damping factor Tan δ . Tg can be determined by the temperature at the peak value of the tan δ curve.
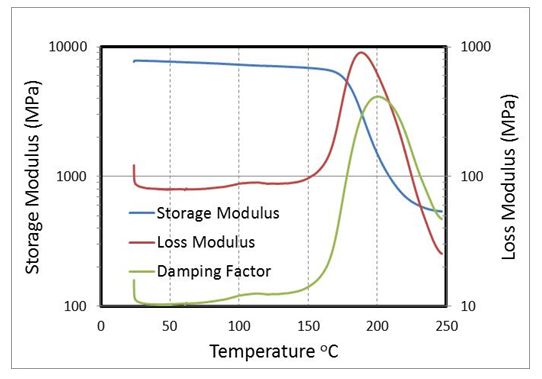 | Figure 10. DMA results for dry sample with 0.2% void content |
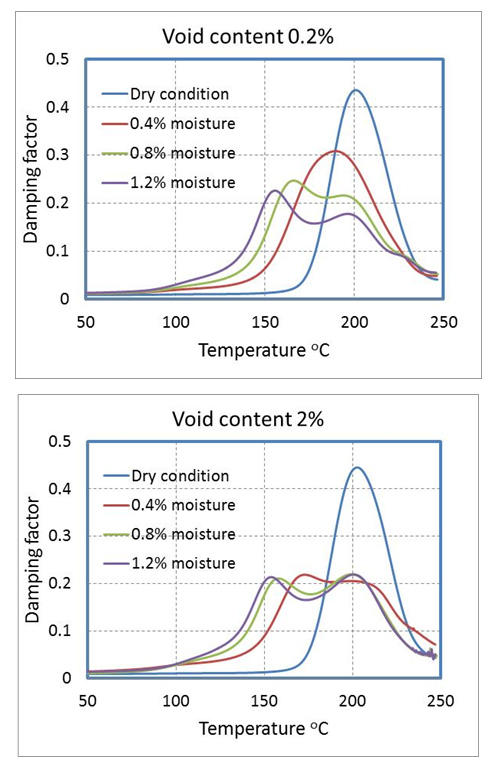 | Figure 11. Variation of Tan δ at different moisture content |
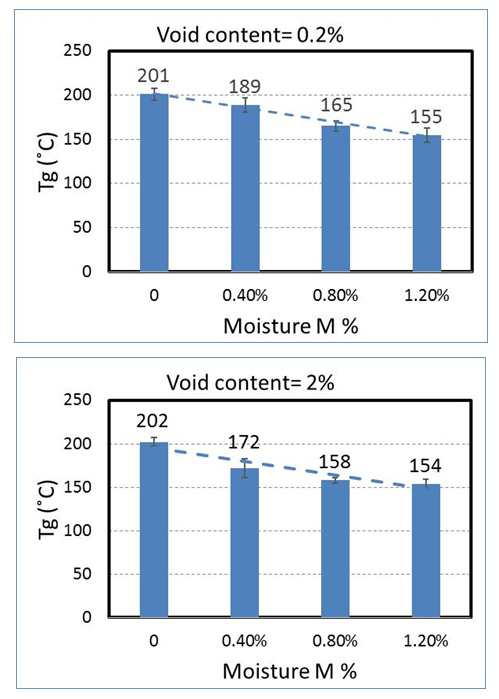 | Figure 12. Change of glass transition temperature with moisture content |
4.4. DiBenedetto Equation
- During the manufacturing of thermosetting composite materials, as the degree of cure and crosslinking increases, the glass transition temperature increases. For many thermosetting systems, the relationship between glass transition temperature Tg and degree of cure (α) can be described by the empirical DiBenedetto equation as [31]:
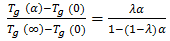 | (7) |
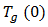 is the glass transition temperature of the uncured resin,
is the glass transition temperature of the uncured resin, 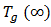 is the glass transition temperature of the fully cured material, and
is the glass transition temperature of the fully cured material, and 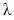 is a material constant. This equation is fitted to the experimental data to obtain
is a material constant. This equation is fitted to the experimental data to obtain 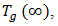
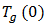 and
and 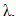 When the thermosets absorb the moisture, the presence of water molecule inside the resin molecular structure has softening effect and therefore the glass transition temperature drops. This behaviour is the opposite to the curing process. However, through analogy, the form of the DiBenedetto equation can be adopted to capture the variation of the glass transition temperature vs moisture content (M). The modified DiBenedetto equation can be expressed as
When the thermosets absorb the moisture, the presence of water molecule inside the resin molecular structure has softening effect and therefore the glass transition temperature drops. This behaviour is the opposite to the curing process. However, through analogy, the form of the DiBenedetto equation can be adopted to capture the variation of the glass transition temperature vs moisture content (M). The modified DiBenedetto equation can be expressed as 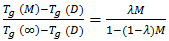 | (8) |
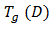 is the glass transition temperature of the completely dried resin,
is the glass transition temperature of the completely dried resin, 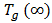 is the glass-transition temperature of the fully saturated resin, and
is the glass-transition temperature of the fully saturated resin, and 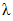 is a material constant. M is the moisture content (%). This equation was fitted to the experimental data to obtain
is a material constant. M is the moisture content (%). This equation was fitted to the experimental data to obtain 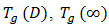 and
and 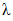 (Figure 13). The results are as follows:
(Figure 13). The results are as follows: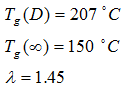 By having the level of moisture, the glass transition temperature can be predicted.
By having the level of moisture, the glass transition temperature can be predicted.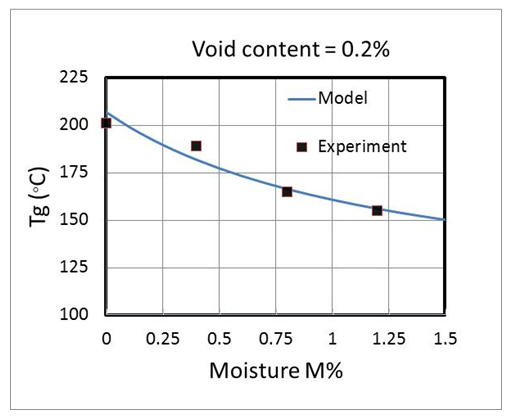 | Figure 13. Variation of glass transition temperature vs level of moisture using proposed modified Dibenedetto equation |
5. Conclusions
- The moisture absorption behaviour of carbon/epoxy unidirectional composite laminates manufactured by out-of-autoclave prepreg material was investigated. Three unidirectional composite laminates containing different levels of void exposed to the hot water at temperature of 60°C. Laminates moisture absorption were monitored by weighting the samples at different intervals. Fick’s law of diffusion was employed to find the moisture diffusivity coefficient. It was shown that as the void content of the sample increased, the diffusivity of the material also increased. A linear equation was also suggested to relate void content of the laminates and diffusivity. Interlaminar shear strength (ILSS) of the laminates was studied by short beam shear tests. The moisture effect on ILSS was almost the same for all levels of void contents. Moisture reduced the ILSS drastically by about 15% compared to the dry condition. Glass transition temperatures (Tg) for all the laminates were determined by the temperatures related to the peak value of tan δ graph from DMA experiments. It was shown that the moisture generally reduced the glass transition temperature of the laminates. Tg tends to reduce after each moisture absorption level. In all levels of void content, Tg reduced by almost 25% after absorbing 1.2% moisture, compared to the dry condition. DiBenedetto equation was proposed and used to correlate the glass transition temperature to the moisture content.
ACKNOWLEDGEMENTS
- The authors gratefully acknowledge the financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML