-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2018; 8(4): 91-96
doi:10.5923/j.cmaterials.20180804.02

Effect of Stearic Acid and Titanate Coupling Agent Modified Calcium Carbonate on Mechanical Properties of Flexible Polyurethane Foam
Ganiyu K. Latinwo1, Olabanji R. Ogunleye1, Samuel E. Agarry1, Ebenezer O. Dada1, Idowu A. Tijani2
1Department of Chemical Engineering, Ladoke Akintola University of Technology, Ogbomoso, Nigeria
2Department of Chemical Engineering, University of Ilorin, Ilorin, Nigeria
Correspondence to: Ganiyu K. Latinwo, Department of Chemical Engineering, Ladoke Akintola University of Technology, Ogbomoso, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Surface modifications of calcium carbonate as filler in flexible polyurethane foams were investigated. In order to improve the dispersion and interfacial bonding between the calcium carbonate and polyurethane matrix, the surface of calcium carbonate was modified using 0.5 wt% stearic acid and 2.72 wt% titanate coupling agent. The influence of modification on the surface of calcium carbonate was characterized with FT-IR and SEM. The mechanical properties of flexible polyurethane foam containing various amount of modified and unmodified calcium carbonate were evaluated by measuring the indentation force deflection, compression set, tensile strength and elongation at break. It was found that the modified calcium carbonate became organophilic as a result of modification. Addition of modified calcium carbonate to polyurethane foam increased the tensile strength while, the compression sets, elongation at break and foam hardness decreased.
Keywords: Stearic acid, Titanate, Flexible polyurethane foam, Calcium carbonate, Spectroscopy
Cite this paper: Ganiyu K. Latinwo, Olabanji R. Ogunleye, Samuel E. Agarry, Ebenezer O. Dada, Idowu A. Tijani, Effect of Stearic Acid and Titanate Coupling Agent Modified Calcium Carbonate on Mechanical Properties of Flexible Polyurethane Foam, International Journal of Composite Materials, Vol. 8 No. 4, 2018, pp. 91-96. doi: 10.5923/j.cmaterials.20180804.02.
Article Outline
1. Introduction
- Flexible polyurethane foams are open cell structure polymer material produced by reaction of diisocyanate with polyols and other low molecular weight materials as additives. The major raw materials (polyol and isocyanate) for the production of flexible polyurethane foam are petrochemical based and highly costly resulting in exorbitantly priced flexible polyurethane products. In order to reduce cost and improve some base property such as surface hardness, calcium carbonates found abundantly in rock are used as filler in foam formulation. However, calcium carbonate is inorganic and due to its hydrophilic surface which renders it incompatible with hydrophobic surface of polyurethane, it agglomerates and do not form uniform dispersion in polymer moieties [3]. Manifestation of the inadequacies results in polyurethane foam with poor mechanical properties [10]. To reduce agglomeration and improve dispersion of calcium carbonate in foam matrix, the surface of the inorganic material can be made organophilic through modification by addition of organic compounds (surface modifiers) such as titanate, fatty acids, silane, salicylates, phosphonates etc. Surface modification improves impact strength and aspect ratio of filler. It prevents phase separation and enhances interfacial bonding with chemically dissimilar polymer matrix [5]. In the present work, the surface of calcium carbonate was modified using stearic acid and titanate coupling agent and the influence of modification on the surface of the filler was investigated using FTIR and SEM. The effect of modified filler on the mechanical properties of medium density flexible polyurethane foam was studied.
2. Experimental
2.1. Materials
- Mineral calcium carbonate with average particle size of 3.5μm was purchased from Success Glister Limited. The calcium carbonate was dried in an oven at 150°C for 10 h to remove moisture. Titanate coupling agent: Bis [2-[(2-minoethyl)amino]ethanolato][2-[(2-aminoethyl)amino]ethanolato-O](propan-2-olato) titanate was purchased from Feidianchem, China. Stearic acid, Isopropyl alcohol, ethanol and methanol were obtained from Labtrade. Polypropylene glycol (PPG), Toluene Diisocyanate (80/20), Amine catalyst (TEGOAMIN* BDE), Silicone oil (TEGOSTAB) and Tin catalyst (KOSMO* 29) were obtained from Unifoam, Ilorin, Nigeria.
2.2. Surface Modification of Calcium Carbonate
2.2.1. Modification Using Titanate Coupling Agent
- Calcium carbonate was modified with titanate coupling agent using wet solution method [1]. 35g of calcium carbonate and 100 ml of isopropyl alcohol were stirred in a beaker for 10 min. A dispersed solution of 0.952g titanate coupling agent (2.72 wt%) in isopropyl alcohol was added dropwise into calcium carbonate mixture under continuous stirring for 30 minutes. The mixture was dried in an oven for 24 h at 100°C. The resultant product was denoted as T-GCC.
2.2.2. Modification Using Stearic Acid
- Calcium carbonate was modified with stearic acid using wet solution method [8]. 0.175 g (0.5 wt%) of stearic acid and 100 ml of ethanol were mixed and stirred in a 250 ml beaker for 10 min at 68°C. 35 g of dried GCC was subsequently added under continuous stirring for 30 min. The mixture was dried in an oven at 60°C for 24 h. The resultant product was denoted as S-GCC. FT-IR spectroscopy was carried out to determine the presence of organic layer on the surface of the modified calcium carbonate. Samples were prepared by mixing small quantity of modified and unmodified calcium carbonates with potassium bromide (KBr) which were then compressed into thin pellets. The IR spectra were recorded between the ranges of 4000 - 400 cm-1.Scanning electron micrographs were examined to study the cell morphology of modified and unmodified calcium carbonates. The samples were treated with liquid nitrogen and then coated with gold. Images were taken on the scanning electron microscope (SEM) operated in the secondary electron mode at a 15 kV accelerating voltage.
2.3. Preparation and Production of Flexible Polyurethane Foam
- Modified (S-GCC and T-GCC) and unmodified (GCC) filler reinforced flexible polyurethane foams were synthesized with the amounts of each chemical component chosen to obtain a target density of 24 kg/m3. The amount of filler in the foam formulations were varied from 15 to 25 php as shown in Table 1. The foam was produced using one shot technique. The mixture of polypropylene glycol and filler were stirred in a plastic bucket until there is complete homogenization. Silicone oil, water, Tin and amine catalyst were added simultaneously to the mixture and stirred continuously for 10 min. Toluene diisocyanate was then added to the mixture and the stirring continued for 10 s. The resulting mixture was poured immediately into a mould (22 × 20 × 17 in). After 10 min, the foams were removed from the mould and left to cure for 7 days.
|
3. Results and Discussion.
3.1. Evaluation of Surface Modification Using FT-IR and SEM
- Figure 1 shows the FT-IR spectra of unmodified calcium carbonate (GCC), stearic acid modified calcium carbonate (S-GCC) and titanate coupling agent modified calcium carbonate (T-GCC). The spectra of S-GCC show absorption band at 3293.93 cm-1 which is attributed to stretching vibrations of O-H group. The peak at 1090.24 cm-1 is that of an ester C-O bond [7]. The bond was created as a result of reaction between the stearic acid with free hydroxyl group present on the surface of the calcium carbonate. The absorption peak appearing at 2917.15 cm-1 and 2817.49 cm-1 are attributed to C-H asymmetric and symmetric of methylene group [13]. The characteristic asymmetric stretching appearing at 2359.89 cm-1 is ascribed to CO2 [17]. The spectra of T-GCC show a broad band at 3439.56 cm-1 which is due to stretching vibration of N-H groups [4]. The N-H is from the amino substrate which is part of organo-functional fragment of the titanate coupling agent used for modifying the calcium carbonate. The weak peak around 1302.37cm-1 is attributed to C-O-Ti vibration [6]. Figure 2 shows the cell morphology of the modified and unmodified calcium carbonate. The S-GCC and T-GCC cells are densely packed with less porosity compared to the GCC with particles that are loosely packed and of high porosity. With these characteristics, it is clear the surfaces of S-GCC and T-GCC have been modified.
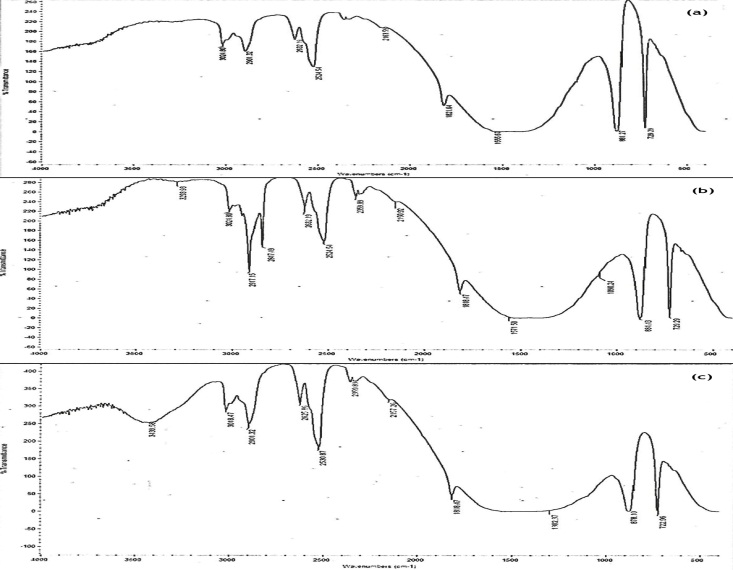 | Figure 1. FT-IR spectra of (a) GCC (b) S-GCC (c) T-GCC |
 | Figure 2. SEM Image (a) GCC (b) S-GCC (c) T-GCC |
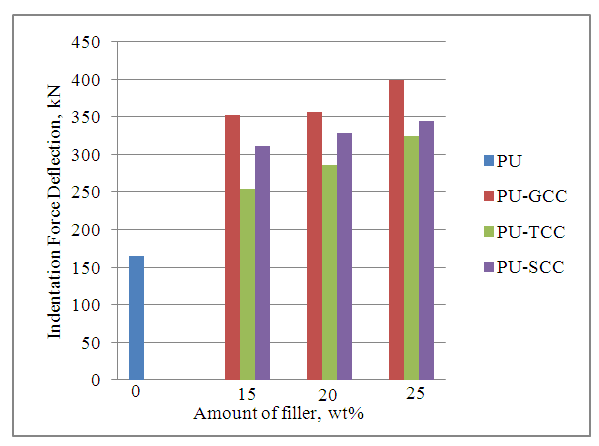 | Figure 3. Effect of filler on the 65% Indentation force deflection |
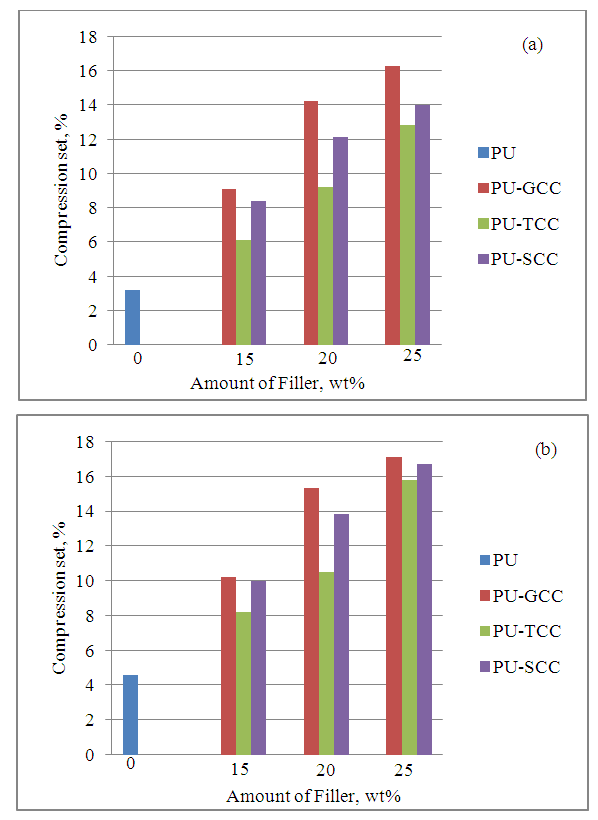 | Figure 4. Effect of filler on compression set (a) at room temperature (b) at heat aging |
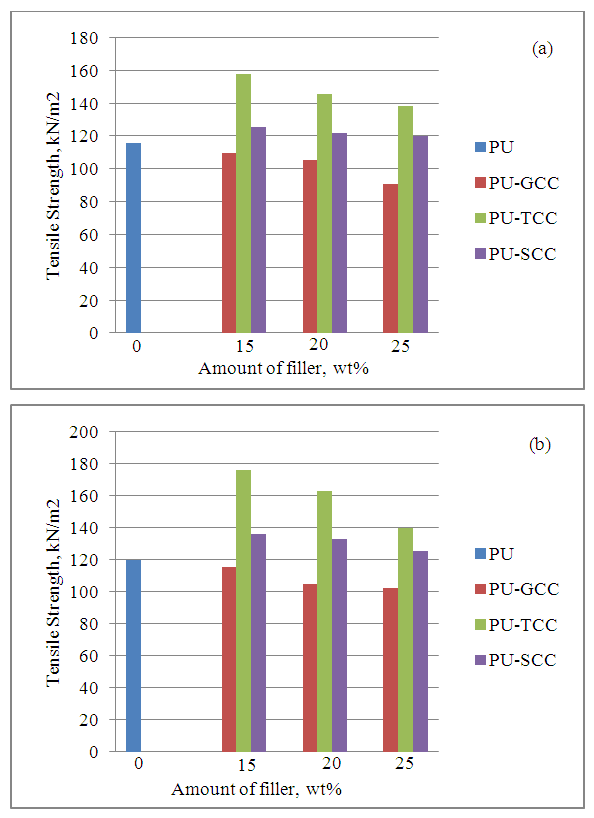 | Figure 5. Effect of filler on tensile strength (a) at room temperature (b) at heat aging |
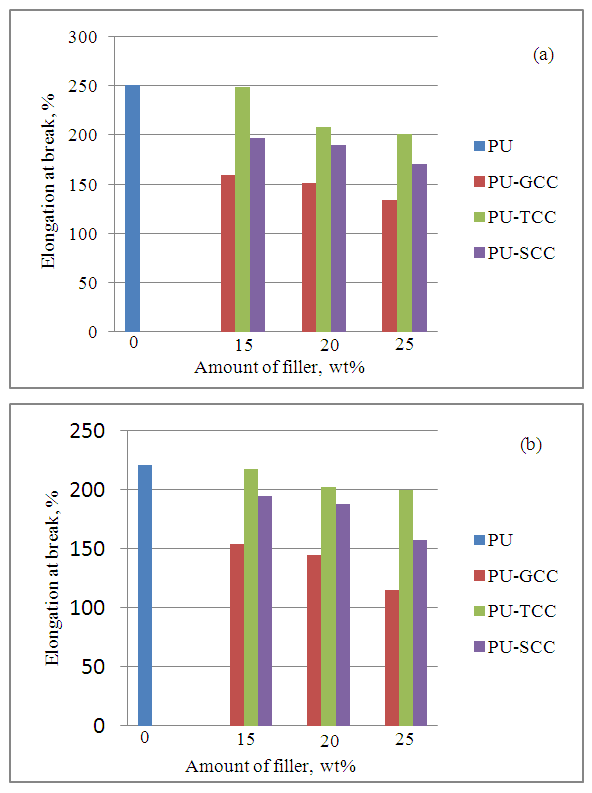 | Figure 6. Effect of filler on elongation at break (a) at room temperature (b) at heat aging |
4. Conclusions
- In this study, ground calcium carbonate (GCC) particles were modified with 0.5 wt% stearic acid and 2.72 wt% titanate to render the GCC surface hydrophobic via wet solution methods. Flexible polyurethane /GCC composite foams containing various amount of modified and pristine GCC were prepared using a one-shot foaming technique. FT-IR patterns showed presence of ester C-O on the S-GCC which was formed from the reaction of stearic acid with the free OH group on the surface of the GCC, and the presence of C-O-Ti which precipitated on the surface of T-GCC particles forming a discontinuous coating layer. It was also shown through SEM that the S-GCC and T-GCC contained densely packed cells compared to pristine GCC. Mechanical properties of the polyurethane foam samples were affected by the incorporation of GCC in such a way that the IFD increased with increase in GCC loadings for the PU-GCC, PU-SCC and PU-TCC. Compared to pristine PU, all the reinforced PU show improved IFD. Modification of the GCC to some extent have appreciable influence on the IFD of PU foam, although IFD of PU-GCC was superior to that of PU-SCC and PU-TCC. Compression sets were affected in a similar manner for the room temperature and heat ageing conditions. Tensile strength of the pristine PU foam was good and those of the reinforced PU foam with GCC, modified or not, decreased with increase in GCC loadings. Among the reinforced PU foam, T-GCC has the best tensile strength property. Similar trend as of tensile strength was observed for the elongation at break for the foam samples. In this regard, one can conclude that the modified calcium carbonate, having better dispersion in PU foam matrix improve the mechanical properties of PU/GCC composite foam.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML