-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2018; 8(1): 10-17
doi:10.5923/j.cmaterials.20180801.02

Dispersion of MWNT under Different Solvents and Its Effect on the Electrical Properties of Cured-Epoxidized Linseed Oil Composites
E. Vigueras Santiago1, M. A. Camacho López1, J. E. Moreno Marcelino2, S. Hernández López1
1Laboratorio de Desarrollo y Caracterización de Materiales Avanzados (LIDMA); Facultad de Química de Química de la Universidad Autónoma del Estado de México (UAEM). Paseo Tollocan Esquina con Paseo Colón, s/n. Moderna de la Cruz, Toluca
2Student in the Materials Science Master Program, UAEM
Correspondence to: S. Hernández López, Laboratorio de Desarrollo y Caracterización de Materiales Avanzados (LIDMA); Facultad de Química de Química de la Universidad Autónoma del Estado de México (UAEM). Paseo Tollocan Esquina con Paseo Colón, s/n. Moderna de la Cruz, Toluca.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Non-functionalized and functionalized multiwalled nanotubes (MWCNTs) were dispersed in epoxidized linseed oil (ELO) using an ultrasonic bath. Qualitative dispersion of the three different carbon nanotubes (CNTs) in four different solvents with and without ELO was followed by optical microscopy, and quantitative analysis of CNTs was performed by calculating the critical concentration from their percolation curves after they were thermally cured. Dimethyl formamide (DMF) was found to be a better solvent for dispersing the three kinds of MWCNTs, but some re-agglomeration of the functionalized MWCNTs occurs upon the addition of ELO. This effect had a weak impact, with the critical concentration of the polymer composites (PCs) being 18% higher for those prepared with functionalized MWCNTs than for those prepared with non-functionalized MWCNTs.
Keywords: Multiwalled carbon nanotubes, Electrical percolation, Nanoparticles dispersion, Epoxidized linseed oil
Cite this paper: E. Vigueras Santiago, M. A. Camacho López, J. E. Moreno Marcelino, S. Hernández López, Dispersion of MWNT under Different Solvents and Its Effect on the Electrical Properties of Cured-Epoxidized Linseed Oil Composites, International Journal of Composite Materials, Vol. 8 No. 1, 2018, pp. 10-17. doi: 10.5923/j.cmaterials.20180801.02.
Article Outline
1. Introduction
- Multiwalled nanotubes (MWCNTs) are some of the most widely used nanoparticles for reinforcement in order to improve the mechanical [1, 2], thermal, tribological [1, 3, 4], optical and electrical properties of polymer matrices [5, 6]. In particular, recent work has showed that an appropriate dispersion of the particles is especially important for obtaining a low concentration threshold [5, 7]. However, obtaining a stable and homogeneous dispersion of MWCNTs into the polymer matrix is one of the main challenges in the production of MWCNT-based polymer composites. Good dispersion of CNTs in polymer matrices can be achieved by means of high power dispersion, compatibilizers, polymer-assisted melt blending [3, 8], and surfactants, resulting in the enhancement of the mechanical and thermal properties. [4, 8-10]. Physical and chemical functionalization of MWCNTs is also one of the most used strategies due to its relative simplicity and efficiency for improving the MWCNT dispersion through better interactions with the matrix polymer [1, 5, 9-14]. However, depending on the degree and nature of functionalization, they could degrade the electrical properties of MWCNTs as demonstrated by Park et al. [15], Lau et al. [10] and Moreno Marcelino [17]. Epoxy resin is a cross-linked polymer extensively used as the matrix for advanced composites [6, 11, 12] due to its good specific strength, stiffness, chemical resistance, and dimensional stability. Because of their high stiffness and strength, resistance to chemicals, good dielectric behavior, resistance to corrosion and microbial organisms, low shrinkage during curing and good thermal features, epoxy resins are the most important class of thermosetting resins with multiple engineering applications. Thus, several research efforts are currently being pursued for the development of the reinforcement of epoxy matrices with CNTs. Currently, vegetable oil-based polymers have attracted attention due to their feasibility for the production of polymers and composites that are environmentally friendly with a great variety of reinforcement particles [18-22]. Epoxidized linseed oil could be easily synthesized from linseed oil and hydroperoxy acid using several well-studied methods [23-27]. The obtained ELO could have an average of 6.0 oxirane rings per molecule and is a nonvolatile and non-polar liquid that could be thermally cured to make it a crosslinked, insoluble and non-processable resin. It could form stable films on several substrates, and it could be mixed with a great variety of fillers, including carbon nanoparticles. Most studies have focused on the evaluation of parameters such as type, size [28], functionalization, geometry, and purity of the MWCNT, with the studies of multiple properties, mainly mechanical, rheological [29] and thermal properties. One approach for evaluating a set of the preparation parameters that provide a better dispersion of the conductive particles is to evaluate their resistivity. Most authors use the lowest resistance as the value that corresponds to the best dispersion conditions [30]. However, Ref. 8 demonstrated that the reproducibility of the resistivity (lower standard deviation) is a better parameter for determining the best dispersion. This was also evidenced by the lower critical concentration results [31]. It is possible that MWCNTs functionalized with polar groups such as hydroxyl and carbonyl groups from carboxylic acids could provide a better dispersion in ELO due the interactions among these functional groups on MWCNTs and with functional groups of the ELO such as carbonyl groups from esters and oxygen from oxirane rings. In this work, we prepared polymer composites with non-functionalized and functionalized-MWCNTs under the same conditions in order to compare their ability for dispersion in ELO and in different solvents. Optical microscopy was used to provide qualitative support for visual tracking of the dispersion process, which was in agreement with the critical concentration results.
2. Methodology and Experimental
2.1. Reactants and Equipment
- MWCNT of D x L: 110-170 nm x 5-9 µm and density (25°C) 1.7-2.1 g/cm3 were supplied by Sigma-Aldrich Co., USA. The MWCNTs underwent three oxidant reactions, and their surface and electrical properties were characterized [17]. Unmodified (MWNT0) and two modified MWCNTs (MWCNT1 and MWCNT2) were chosen for the preparation of the polymer composites. Epoxidized linseed oil (ELO) was prepared by the chemoenzymatic method [32], achieving 98% epoxidation and molecular mass of 980.4 g/mol as determined by H1NMR. N,N-dimethylformamide (DMF), acetone (Ac), tetrahydrofuran (THF) and ethyl acetate (AcEt) with analytical purity were supplied by Sigma-Aldrich, Co. All of these reagents were used as received. For dispersion, an Ultrasonic 20X-NEY instrument was used at the frequency of 40 Hz. For characterization, an Olympus BX-41 optical microscope was used to track the dispersion of the different MWCNTs into ELO with the different solvents. DSC SDT Q600 modulus instrument at temperatures ranging from 30 to 300°C under nitrogen gas (100 mL/min) was used for setting curing conditions; an FTIR Prestige 21 instrument in the HART mode was used to corroborate the total curing of the composites by following the disappearance of the signal at 820-800 cm-1, which corresponds to the epoxy ring vibration. An ADP 21-Yamato programmable vacuum drying oven was used for curing the composites; a digital multimeter MUL-600 was used for measuring resistances lower than 40 MΩ, while a Keithley electrometer 6517A was used for the measurements of higher resistances. The SEM images were taken at 2,500x using a JEOL JSM-6510LV instrument with voltages of 25 and 30 kV and tungsten filament, and it was not necessary to cover the sample surfaces with a metallic conductive layer. SEM was used to show the dispersion of the MWCNTs into the cured composite.
2.2. Dispersion Evaluation of the Different MWCNTs in Different Solvents by Optical Microscopy
- The ultrasonic dispersions of MWCNT in the four solvents were evaluated by optical microscopy at 10x, 50x and 100x and at different times. A general procedure consisted of the preparation of 3 mg/mL of MWCNT0, MWCNT1 and MWCNT2 in DMF in three different tubes, which were then dispersed by sonication at 40 Hz at room temperature (23°C) in order to follow the dispersion. A drop of the dispersed solution was taken and dropped into glass substrate and then analyzed under the optical microscope every hour until 24 h. The analysis was only visual, and the dispersion time was recorded as the time when some individual MWCNTs were observed without an important enhancement with longer times. The experiment was repeated at least three times in order to provide reliable dispersion time values. The dispersion times varied depending on the type of MWCNTs and the solvent. After the dispersion was reached, 40 mg of ELO/mL was added in order to obtain the composition with 7% w/w MWCNTs. Some instantaneous agglomeration was observed during this addition, which was due to the dilution of the ELO changing the interactions with the MWCNTs. All mixtures were dispersed in an ultrasonic bath following the same procedure.
2.3. Composites Preparation
- Using the aforementioned conditions, the same two-stage procedure was followed for the preparation of 2 mL of other compositions: 1.5, 2, 2.5, 3, 4, 5, 6, 8 and 10% w/w of MWCNT. In all cases, the same concentration of 3 mg/mL of MWCNT was maintained. After dispersion, the solvent was quickly evaporated by distillation under reduced pressure. The viscous slurry was extended on at least three previously pulled and washed brass substrates with the dimensions of 2x2 cm. Then, the slurries were cured in a programmable oven at two temperatures: 170°C for 10 min in order to eliminate the residual solvent and avoid bubbles and at 210°C for 1.5 h to complete the crosslinking. Polymerization was confirmed by FTIR spectroscopy and DSC. Thus, films with 0.02 to 0.05 mm thickness were obtained, which were then covered with electrodes by painting their surfaces with conductive silver paint. Finally, the resistance of each layer was measured using a digital multimeter MUL-600 instrument for resistances lower than 40 MΩ, while for higher resistances, a Keithley electrometer 6517A instrument was used, and the resistivities of the samples were calculated. The obtained data were fit to the nonlinear percolation equation [34-36]. It was found that the three kinds of MWCNTs were better dispersed in DMF. Analysis by optical microscopy only provided support for determining the minimum time for establishing a dispersion of the MWCNT in a solvent and in the solvent + ELO solution. The dispersion was considered to be better when some individual MWCNTs were observed in conjunction with fewer agglomerates and the minimal time when this state does not change at longer dispersion times. The dispersion time varied depending on the MWCNT type. Whereas MWNT0 require 4 h for reaching better dispersion, modified MWCNTs only needed 2 h and 1 h, respectively (Table 1).
3. Results and Discussion
- The evaluation of CNT dispersion in a solvent is not trivial due to the non-transparency even at a very low CNT concentration and due to the absence of reliable methods for the evaluation of the dispersion quality [10, 31]. One of the most used procedures in order to evaluate the better dispersion of CNT was described in [31] where TEM, SEM, optical microscopy and a theoretical analysis based on an analytical model of an interparticle distance concept were used to correctly reflect the different dispersion states, namely, entangled bundles and well-dispersed individual CNTs, of the CNTs in the matrix.CNT–epoxy nanocomposites with different dispersion states have been fabricated from the same constituent materials by employing different processing conditions. However, these studies were performed on the final composites. Our proposal was to develop a method for the easy tracking of the solution dispersions before the curing step that corresponded to the percolation thresholds of the nanocomposites. This is because optical microscopy [31] was used as an available, quick and easy tool to follow “in real time” the dispersion of the particles in solution (without and with ELO) in order to save time and decrease the number of the samples that must be prepared before the curing process. It is important to note that these results are in agreement with and provide an explanation for the results obtained from the electrical characterization of the cured composite materials. Figure 1 shows an example of optical images obtained for MWNT0 with the four solvents (from the top the bottom: Ac, AcEt, THF and DMF) at the same time (4 h) and at amplifications of 10x, 50x and 100x (from the left to the right). It can be seen that at 10x, the first three series of the images from top to bottom show agglomerates of MWCNT (dark zones), whereas the last series of images (DMF) show a much better homogeneous dispersion. A closer view at 100x shows the bundles of MWCNTs only covering some region of the surface, while in DMF, the MWCNTs are dispersed in the entire selected region, conveniently forming a continuous pathway with smaller agglomerates. This analysis was then repeated for the sample with added ELO. Unfortunately, after the addition, some re-agglomeration of the MWCNTs was observed, and two phases could be distinguished when DMF was the solvent. Figure 2 shows the evolution of the mixtures at 1, 2, 3, and 4 h of sonication after the addition of the ELO to the MWCNT solution in DMF. For the first two hours, the agglomeration of the carbon particles and the two phases are evidenced. For the third hour, the mixture appears homogeneous and the MWCNTs reach their maximal dispersion, while for the fourth hour, the MWCNT dispersion does not improve, but some separation phase could be observed. This is due to the three additional hours of sonication of the mixtures after the addition of the ELO. The values of the total dispersion time for each composite are presented in Table 1.
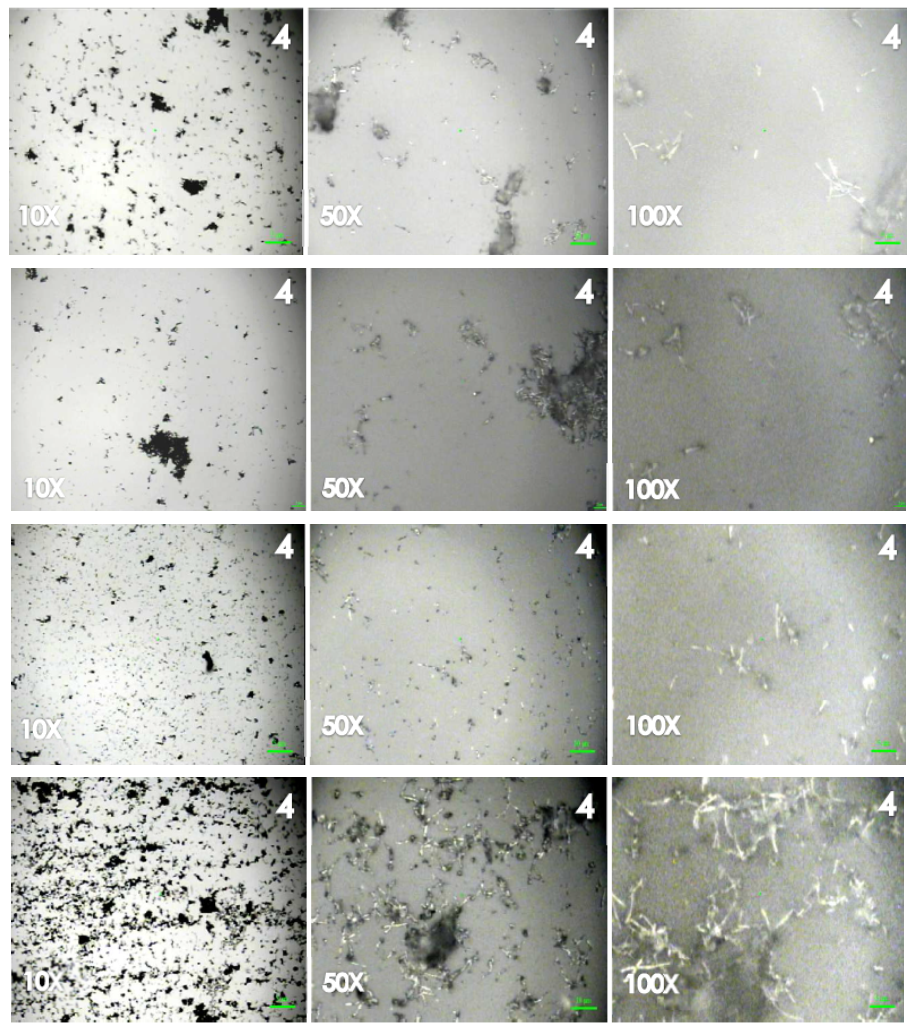 | Figure 1. Dispersion of the MWNT0 in different solvents: Ac, AcEt, THF and DMF from top to bottom, all of them taken at 4h of sonication |
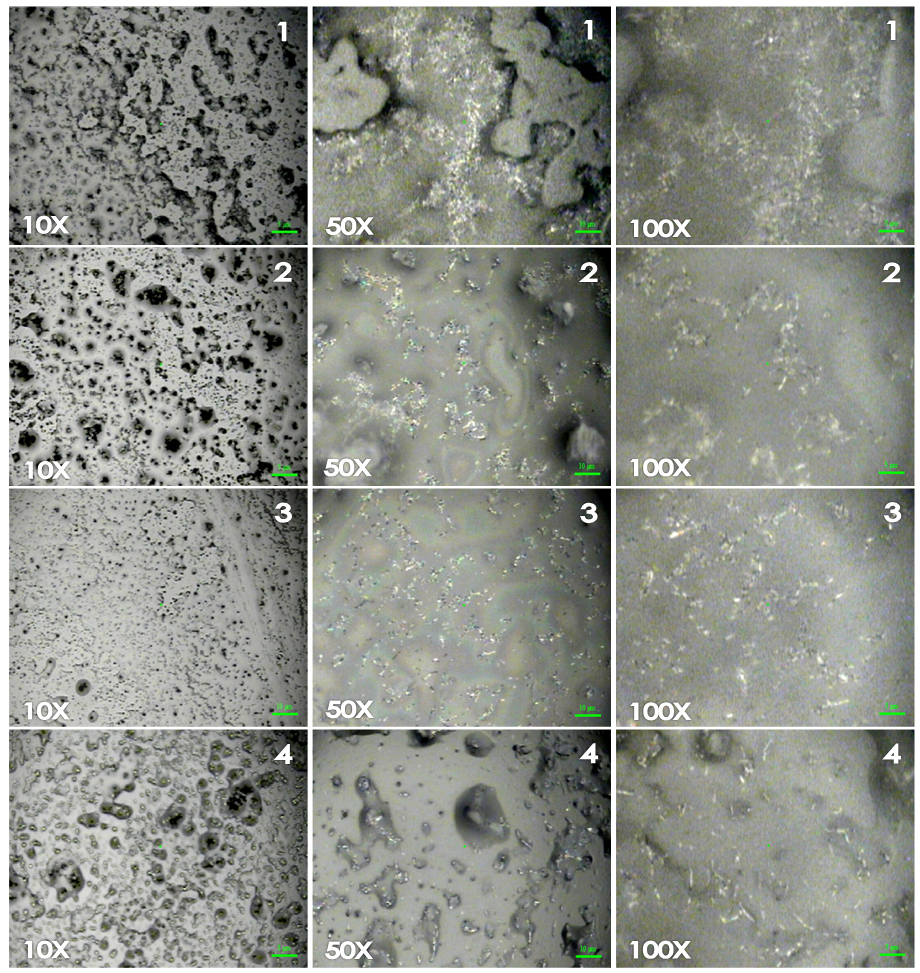 | Figure 2. Dispersion evolution of the MWNT in DMF after adding ELO, images were taken at 1, 2 3 and 4 h of sonication |
|
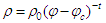 | (1) |
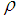 is the resistivity of the medium that jumps down several orders of magnitude due to the formation of continuous electron paths or conducting networks.
is the resistivity of the medium that jumps down several orders of magnitude due to the formation of continuous electron paths or conducting networks. 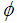 is the CNT weight fraction, and φc is the percolation critical concentration. The parameter t is a critical exponent that governs the scaling law in the vicinity of percolation and has been associated with the dimensionality of the system. Critical calculated concentrations were 2.2, 2.8 and 2.6% wt/wt CB for PC0, PC1 and PC2, respectively. These results could be explained from two points of view. The first is based on the higher electrical resistivity of the CNT after the oxidation in comparison with non-functionalized CNT [14]. If this is the case, the resistivities of PC1 and PC2 should be higher for all compositions with respect to PC0 and showing behavior that is not consistent with the behavior shown in Figure 3. The second hypothesis is based on the occurrence of some re-agglomeration of the functionalized MWNT at the time of the addition of the ELO during the CNT dispersion process. Optical microscopy observed some drops of insoluble ELO in the mixture. DMF was a good solvent for dispersing the MWCNTs, as supported by some other references [37, 38], but not for solvating ELO, which is more soluble in less polar solvents such as toluene, acetone and THF [35, 39]. Even in the case of a post-dispersion process after the addition of the ELO to the mixture of solvent + MWCNT, the polarity of the medium was changed by the presence of the less polar oil. In this case, the functionalized MWCNTs with polar groups on the surface (MWCNT1 and MWCNT2) modified their ability for dispersing in the medium and preferred to interact with each other, forming small agglomerates and therefore increasing the amount of particles required to achieve conductivity. The second supposition explains very well the critical concentration results and the percolation curves shown in Figure 3.To verify the uniform distribution of individual CNTs on the microscopic scale in cured composites [35, 39], two PC0 compositions, 4 and 6% wt/wt of MWCNT0, were analyzed by SEM (Figures 4(a) and (b), respectively). In both cases, it is possible to distinguish the MWCNTs dispersed mainly individually and less so in small agglomerates. This dispersion was better observed for 4 and 6% MWCNT0 that are both above the critical concentration. It is important to mention that the electrical measurements were reproducible with the standard deviation shown by the errors bars in Figure 3.
is the CNT weight fraction, and φc is the percolation critical concentration. The parameter t is a critical exponent that governs the scaling law in the vicinity of percolation and has been associated with the dimensionality of the system. Critical calculated concentrations were 2.2, 2.8 and 2.6% wt/wt CB for PC0, PC1 and PC2, respectively. These results could be explained from two points of view. The first is based on the higher electrical resistivity of the CNT after the oxidation in comparison with non-functionalized CNT [14]. If this is the case, the resistivities of PC1 and PC2 should be higher for all compositions with respect to PC0 and showing behavior that is not consistent with the behavior shown in Figure 3. The second hypothesis is based on the occurrence of some re-agglomeration of the functionalized MWNT at the time of the addition of the ELO during the CNT dispersion process. Optical microscopy observed some drops of insoluble ELO in the mixture. DMF was a good solvent for dispersing the MWCNTs, as supported by some other references [37, 38], but not for solvating ELO, which is more soluble in less polar solvents such as toluene, acetone and THF [35, 39]. Even in the case of a post-dispersion process after the addition of the ELO to the mixture of solvent + MWCNT, the polarity of the medium was changed by the presence of the less polar oil. In this case, the functionalized MWCNTs with polar groups on the surface (MWCNT1 and MWCNT2) modified their ability for dispersing in the medium and preferred to interact with each other, forming small agglomerates and therefore increasing the amount of particles required to achieve conductivity. The second supposition explains very well the critical concentration results and the percolation curves shown in Figure 3.To verify the uniform distribution of individual CNTs on the microscopic scale in cured composites [35, 39], two PC0 compositions, 4 and 6% wt/wt of MWCNT0, were analyzed by SEM (Figures 4(a) and (b), respectively). In both cases, it is possible to distinguish the MWCNTs dispersed mainly individually and less so in small agglomerates. This dispersion was better observed for 4 and 6% MWCNT0 that are both above the critical concentration. It is important to mention that the electrical measurements were reproducible with the standard deviation shown by the errors bars in Figure 3. 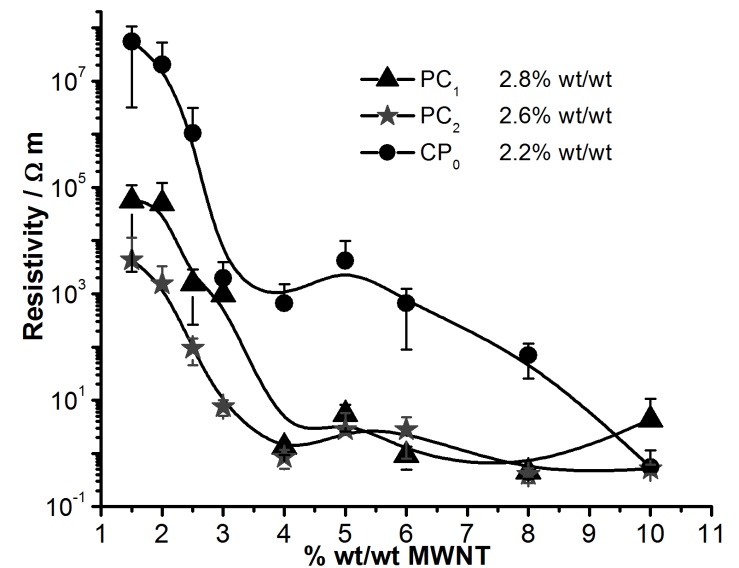 | Figure 3. Percolation curves for the three series of polymer composites: PC0, PC1 and PC2 |
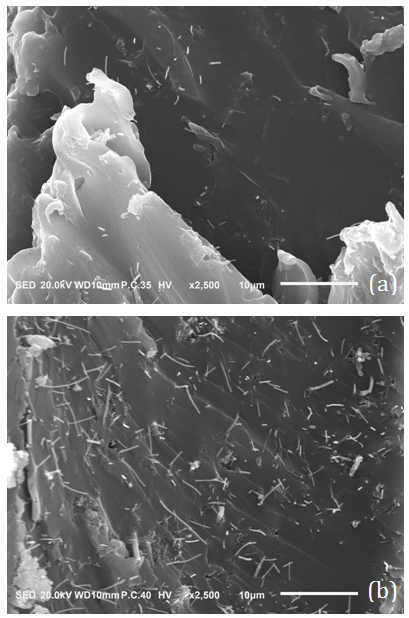 | Figure 4. SEM images for PC0 at (a) 4 and (b) 6% wt/wt of MWCNT0 |
4. Conclusions
- Optical microscopy was used for establishing a procedure for following the dispersion of different MWCNTs in solution during sonication at different times. This monitoring process in practically "real time" allows or the limiting of the processing time of the MWCNTs in solution when different solvents were used. It also allowed for the observation of the re-agglomeration process of CNTs when the polarity of the medium in which they were already disentangled (DMF) was modified by the addition of the ELO. This phenomenon of re-agglomeration by polarity change or change in any other parameter such as temperature could be present but is no detected by other techniques nor examined in the final cured composites, and it is completely ignored. Thus, this tracking technique could be useful for the studies of CNT dispersion in solvent mixtures aimed to improve the preparation conditions and to optimize the electrical properties in the final composites. The good agreement with the electrical properties and good reproducibility in the final composites after curing of the dispersed solutions provided evidence for the usefulness of the tracking technique developed in this work.
ACKNOWLEDGEMENTS
- This research was financially supported by project UAEM under the project No. 4314/2017/CI. We thank to Dr. Víctor Castrejón Sánchez from Instituto Tecnológico de Jocotitlan (México) for SEM analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML