S. Javzan1, D. Selenge1, D. Nedelcheva2, V. Christov2, S. Philipov2
1Laboratory of Natural Product Chemistry, Institute of Chemistry and Chemical Techology, Mongolian Academy of Sciences, Ulaanbaatar, Mongolia
2Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences, Sofia, Bulgaria
Correspondence to: S. Javzan, Laboratory of Natural Product Chemistry, Institute of Chemistry and Chemical Techology, Mongolian Academy of Sciences, Ulaanbaatar, Mongolia.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Alkaloids of the aerial parts of P. multisectum (Maxim) Bobrov (Zygophyllaceae) growing in Mongolia have been studied by capillary Gas Chromatography-Mass Spectrometry (GC-MS) and Column Chromatography (CC). Four compounds comprising of 2-methylquinoline (1), 9-amino-2, 3,5,6,7,8-hexahydro-1H-cyclopenta [b] quinoline (2), vasicinone (3) and harmine (4) have been determined by GC-MS, while harmine (4), peganine (5), deoxypeganine (6), deoxyvasicinone (7) and harmane (8) were isolated as pure compounds by the CC. The structures of the five alkaloids 4, 5, 6, 7 and 8 were elucidated by the MS and 1H and 13C NMR analysis. The alkaloids 1, 2 and 8 were identified for the first time from this species.
Keywords:
Peganum multisectum (Maxim) Bobrov, Quinoline, Quinazoline and indole alkaloids, GC-MS
Cite this paper: S. Javzan, D. Selenge, D. Nedelcheva, V. Christov, S. Philipov, Alkaloids from Mongolian Species of Peganum Multisectum (Maxim) Bobrov, International Journal of Composite Materials, Vol. 7 No. 6, 2017, pp. 155-160. doi: 10.5923/j.cmaterials.20170706.01.
1. Introduction
The family of Zygophyllaceae can be considered as Zygophyllales, which contains more than 285 species within 22 genera. The genus of Peganum has six species such as Peganum harmala L., Peganum mexicanum Gray, Peganum nigelalastrum Bunge, Peganum rothschildianum F. Buxbaum, Peganum texanum M. E. Jones and Peganum multisectum (Maxim) Bobrov, which occur in the regions of warm and subtropical temperatures in the world. These species were widely distributed across North Africa, Pakistan, India and Southern part of Iran and have been introduced in Southern and Northern America, Australia, Mexico, Tunisia, China and Mongolia [1-3]. Peganum nigelalastrum Bunge, Peganum harmala L. and Peganum multisectum (Maxim) Bobrov are common plants in Mongolia. In particular, Peganum multisectum recently found for the first time in the Eastern and Alashan Gobi part of Mongolia [4, 5].In 1994, Cai and his coworkers identified total 34 volatile components from the stem and leaves of P. nigellastrum, P. multisectum and P. harmala by GC-MS analysis. The alkaloid fractions of these three plant species showed anti-tumor activities [6-8]. In previous studies, the alkaloids harmine, evodiamine, vasicine, vasicinone, deoxyvasicinone and fagomine were reported from Peganum multisectum [9]. Currently, no results have been found on phytochemical study of P.multisectum grown in Mongolia. This is the first report of the alkaloids study of this species.
2. Experimental
Gas Chromatography-Mass Spectrometry (GC-MS): The GC-MS: The GC-MS, which is equipped with fused silica capillary column 30 m x 0.25mm x 0.25µm, has been used for the identification of 2-methylquinoline (1), 9-amino-2,3,5,6,7,8-hexahydro-1H-cyclopenta[b]quinolone (2), vasicinone (3) and harmine (4).Moreover, the HP-5 MS phase coupled with Hewlett Packard 6890/MSD 5793A E were also applied in this study. Flow rate of the helium with 0.8ml/min was used to carry a gas. A detailed program of the GC-MS as follows: temperature 50-300°C at 6° /min, isotherma 0-10 min, solvent delay 2.0 min and mass range 50-750. In addition, the flame ionization detector was used at Tinl 260°C, Taux 280°C. The molecular weight and structure of compounds from the total alkaloids were ascertained by interpretation of GC-MS using the database of the National Institute of Standard and Technology (NIST) of the US Department of Commerce.Column chromatography (CC): Neutral alumina (Merck, Aluminum oxide 90, act. II-III Brockmann, 70 (230 mesh). Preparative Thin Layer Chromatography (PTLC) and Thin Layer Chromatography (TLC): Kieselgel 60: GF 254. Mobile phases (Mph) used for PTLC are acetone, aceton-methanol and aceton-methanol-ammonia (2:03:1 drop, 2:0.3:2 drops). Visualization for TLC: Dragendorff reagent. General: Mass Spectrometer (MS) Hewlett Packard MSD 5973, 70 eV. 1H and 13C NMR: 219 Bruker DRX-250, in CDCl3, with TMS as an internal standard. GC-MS, 1H, 13CNMR and MS methods were used to determine and describe the structure of alkaloids.Plant material: Aerial parts of P. multisectum (Maxim) Bobrov were collected in June from Sebrei sum of Umnugobi province, Eastern Mongolia. The plant was identified by Prof. N. Amartuvshin, Mongolian Institute of Botany MAS, where a voucher specimen was deposited. Extraction: The air-dried aerial parts of P. multisectum (223.0 g) were extracted three times with 90% of ethanol and combined extracts were concentrated under vacuum to afford a viscous residue (20.6 g). The residue was treated three times with 300.0 mL of 5% HCl and filtrated. Then the acidic solution was basified with concentrated ammonia solution up to pH 10-11 and extracted three times with chloroform (CHCI3). The chloroform extracts were combined and dried over anhydrous Na2SO4, and then evaporated under reduced pressure to give 0.41g of crude alkaloid mixtures (CAMs). Two μl of CAMs from aerial parts of P. multisectum were dissolved in methanol and employed for GC-MS analysis. Although GC-MS was applied to isolate the total of alkaloids and determine four alkaloids the rest of the alkaloids were isolated by CC. The pure alkaloids were prepared after CC of CAMs on silica gel 60 Merck (0.2-0.6 mm) with the mobile phases acetone, methanol, acetone-methanol and methanol gradient. PTLC on mixed fractions was carried out on plates (20x20 cm silica gel GF254 Merck, 1 mm thickness) and acetone-methanol in vapours of NH3 (2:0.1), acetone-methanol-ammonia (2:0.1:1 drop) and (2:0.3:2 drops) of mph were used. The bands were detected under UV light and or visualization by Dragendorff reagent.Isolation of pure compounds: The alkaloids were eluted with acetone and acetone-methanol mixtures, with increasing methanol concentration (1 to 15%), to give fractions PM-1 to PM-32. According to TLC analysis of all fractions with a mobile phase acetone-methanol (2:0.1) and acetone-methanol-ammonia (2:0.1:1 drop, 2:0.3:2 drops) they were combined to give 5 fractions. PTLC with mph (2:0.1:1 drop) was applied to isolate compound 5 (Pm-41 1.04 mg, and Pm-42 0.62 mg), compounds 8, 3, 4 and 7 (Pm-51 1.07 mg, 8, Pm-53 0.79 mg, 3, Pm-54 0.97 mg, 4, Pm-55 2.32 mg, and Pm-62 0.59 g, 7). Then with mph (2:0.3:2 drops) was used to isolate the compounds 7 (0.74 mg) and 8 (0.98 mg) from Pm-8-9 and Pm-10-15 fractions, respectively. The structures of the compounds harmine (4, 0.97 mg), peganine (5, 1.66 mg), deoxypeganine (6, 0.74), deoxyvasicinone (7, 2.91mg), harmane (8, 0.98mg) were elucidated by MS and NMR spectroscopy.
3. Results and Discussion
The phytochemical investigation of the aerial parts P. multisectum grown in Mongolia afforded 8 alkaloids of the quinolone-, quinazoline- and indole-type, respectively. Two of the alkaloids are quinolines as 2-methylquinoline (1) and 9-amino-2,3,5,6,7,8-hexahydro-1H-cyclopenta[b] quinoline (2), four are quinazolines as vasicinone (3), peganine (5), deoxypeganine (6) and deoxyvasicinone (7), and two are the indole-type as harmine (4) and harmane (8), have been identified by GC-MS, MS and 1H, 13C NMR analysis. Alkaloids 1, 2 and 8 were determined for the first time from this species.Amongst them, 9-amino-2, 3, 5, 6, 7, 8-hexahydro-1H-cyclopenta[b]quinolone 2 (11.501%) was identified as the major alkaloid in the alkaloid extract, while compounds 1 (0.791%), 3 (4.785%) and 4 (0.909%) were detected as the minor (Figure 1 and Table 1) by GC-MS.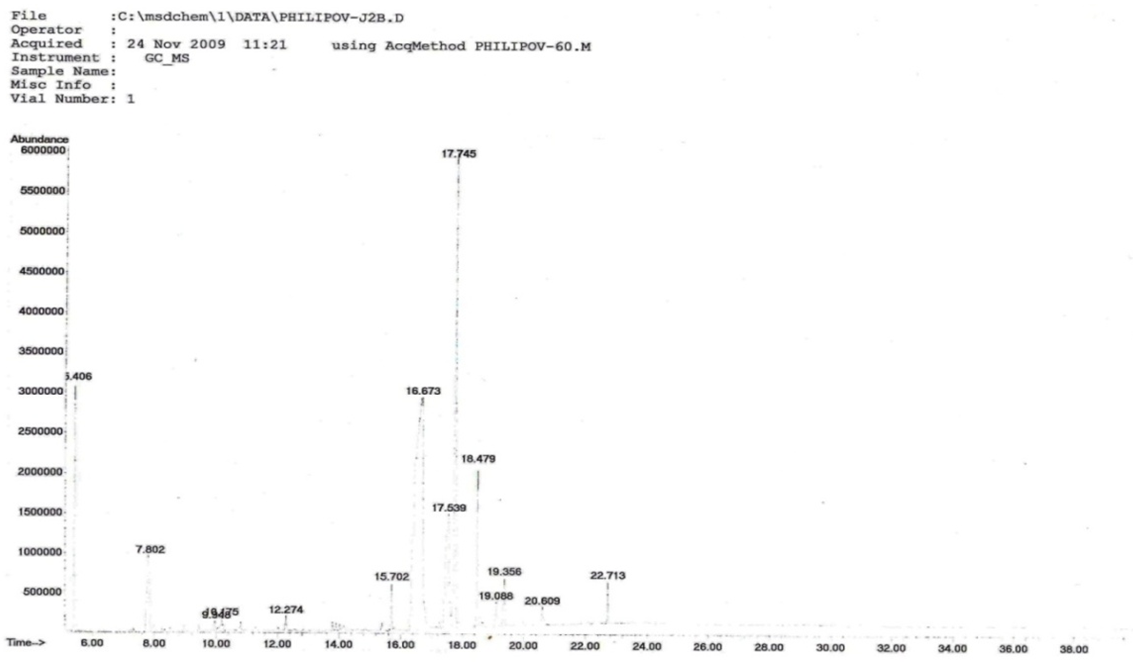 | Figure 1. GC-MS data of aerial parts of P. multisectum |
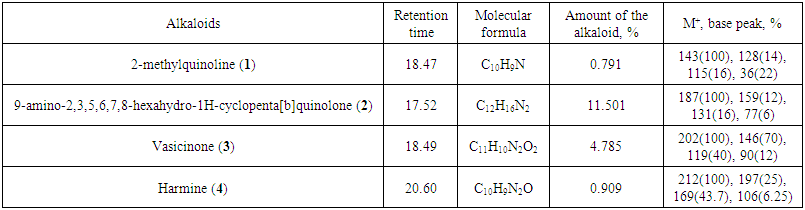 | Table 1. Alkaloids from aerial parts of P. multisectum (Maxim) Bobrov determined by GC-MS analysis |
The structures of the alkaloids were determined based on a comparison of their retention time and mass spectral fragmentation (NIST) with those of reference data in the literature [10].The other five alkaloids were isolated from the aerial parts of P. multisectum by CC. They were analyzed by NMR spectroscopy and their molecular structures were determined as harmine (4), peganine (5), deoxypeganine (6), deoxyvasicinone (7) and harmane (8) (Fig. 2). The assignment of the protons and carbons is described in Table 2-3 and is consistent with the data reported in the previous literature [11, 12].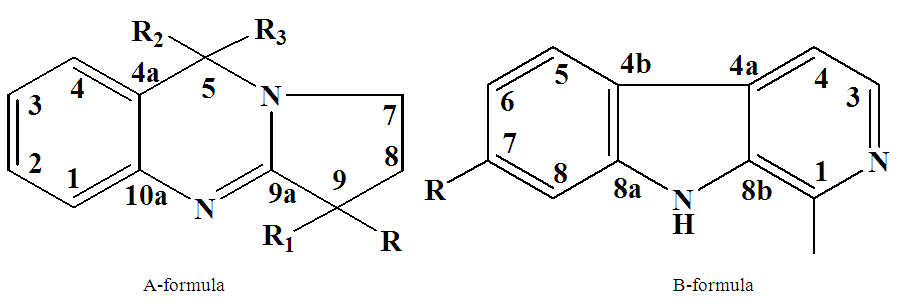 | Figure 2. Molecular structure of the quinazoline-type alkaloids (A-formula): peganine (7,8,9,5-tetrahydro-pyrrolo[8,7-b] quinazoline-9-ol; R=OH, R1=R2=R3=H, 5), deoxypeganine (7,8,9,5-tetrahydro-pyrrolo[8,7-b] quinazoline; R= R1=R2=R3=H, 6), deoxyvasicinone (7.8.9-trihydro-5-oxo-pyrrolo[8,7-b]-quinazoline; R= R1=H, R2=R3=O, 7) and indole-type alkaloids (B-formula): harmine (1-methyl-7-methoxy-β-carboline; R=OCH3, 4) and harmane (1-methyl-β-carboline; R=H, 8) |
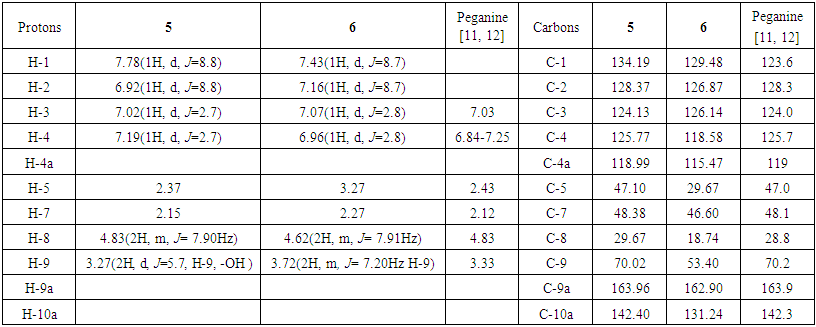 | Table 2. 1H and 13C NMR chemical shift assignment for peganine (5) and deoxypeganine (6) (in CDCl3, δ ppm, J Hz) |
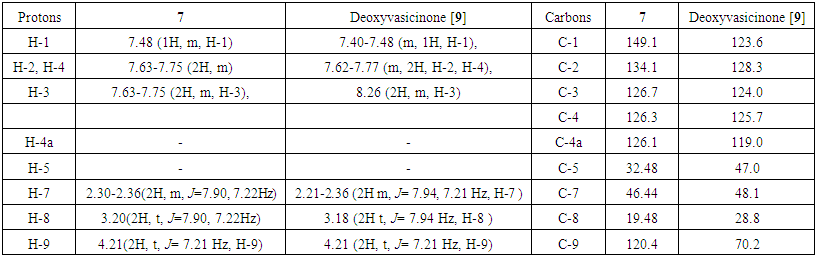 | Table 3. 1H and 13C NMR chemical shift assignment for deoxyvasicinone (7) (in CDCI3, δ ppm, J Hz) |
Peganine (5): The proton NMR chemical shift values for peganine are shown in Table 2. The 1H NMR spectrum of 5 in CDCl3 shows the presence of four doublets at δ, 6.92 (1H, d, J=8.8 H-2), 7.02 (1H, d, J=2.7, H-3), 7.19 (1H, m, J=2.7, H-4) and 7.78 (1H, d, J=8.8, H-1), which were attributed to the four aromatic protons. A multiplet of the hydroxyl group was found at δ 3.27. The chemical shift value at δ 4.18 (1H, t, H-1) confirms the proton which attached to the carbon with OH group. Two multiplets were identified at δ 2.15 (1H, m, H-7) and 2.37 (1H, m, H-8) and are resulting from methylene groups. In addition, one multiplet indicated at δ, 3.27 (1H, m H-9), and a singlet assigned to methylene group appeared at δ, 4.62 (2H, s, H-5). In the carbon spectrum of 5 (Table 2) signals of 11 carbons were determined. A quaternary carbon signals (C-4a, C-9a and C-10a) appeared at δ 118.9, 163.9 and 142.4. The signals at δ 134.19, 128.37, 124.13 and 125.77 were assigned to other aromatic methine carbons of the A-formula. The signals at δ 29.67, 47.10 and 48.38 were attributed to methylene carbons, signal at δ 70.02 were attributed to methine carbon (C-9) of the A-formula.In the MS of 5, the molecular ion peak was determined at m/z 189.5 [M+H]+. Interpretation of the results of 1H and 13C NMR spectral data, and MS let to conclude that the compound is the alkaloid peganine (Fig. 2) [11, 12]. Deoxypeganine (6): It was obtained as a pale white powder, which gives an orange-red color with Dragendorff’s reagent on a TLC plate. A molecular formula was determined as C11H12N2 from the positive-ion molecular peak at m/z 173 [M+H]+. The 1H and 13C NMR spectroscopic data of the alkaloids 5 and 6 were similar, the difference of these two alkaloids was the absence of hydroxyl group in 6. Instead of the hydroxyl group, in 6 appeared signal of methylene group at δ 3.72 (2H, m, H-9). Based on the good agreement between our NMR data with literature [11, 12], 6 was identified as deoxypeganine.Deoxyvasicinone (7): Compound 7 was obtained as a white powder, with the molecular formula C11H10N2O, which resulted from the high-resolution MS analysis m/z [M+1]+ 187. The 1H NMR spectrum of 7 in CDCI3 shows the presence of four multiplets at δ, 7.48 (1H, m, H-1), 7.63-7.75 (2H, m, H-2, H-3) and 7.19 (2H, m, H-4) which were attributed to the four aromatic protons. One multiplet at δ, 2.30-2.36 (2H, m, J=7.90, 7.22 Hz, H-7), and two triplets 3.20 (2H, t, J=7.90, 7.22 Hz, H-8) and 4.21(2H, t, J=7.21, H-9). The 1H and 13C NMR spectroscopic data of the alkaloids 6 and 7 were similar, the difference of these two alkaloids was the absence of a methylene group in 7. Instead of the methylene group in 7 appeared carbonyl group. In the carbon spectrum (Table 2) the signals of 11 carbons were determined. Four quaternary carbon signals appeared at δ 126.17, 32.48, 159.38 and 160.95 in the 4a, 5, 9a and 10a positions. The signals at δ 149.11, 134.10, 126.76 and 126.35 were assigned to other aromatic methine carbons of the A-formula. Three methylene carbon signals appeared at δ 46.44, 19.48 and 120.46 positions of the A-formula (Fig. 2). All evidence confirmed the structure of 7 which was identified as deoxyvasicinone [9].Harmane (8): Compound 8 was obtained as a white powder, with the molecular formula C11H10N2O, which resulted from the high-resolution MS analysis m/z 182. It gave positive Dragendorff’s test for alkaloids. The 1H NMR spectrum of 8 in CDC13 showed signals for ortho-disubstituted phenyl ring at δ, 8.35(1H, d, J=5.2Hz, H-3), 8.05(1H, d, J=5.6Hz, H-4), 8.15(1H, d, J=8.0Hz, H-5) in addition to an at δ, 7.30(1H, ddd, J=6.8Hz, H-6), 7.60(1H, ddd, J=7.2Hz,, H-7) and 7.65(1H, d, J=8.0Hz, H-8) and other proton that appear upfield at δ, δ2.96 (3H, s, -CH3) was assigned to a methyl proton. The 13C NMR spectrum displayed resonances for 12 carbons; including one methyl, six methines, and four quaternary carbons. Quaternary carbon signals appeared at δ 141.6, 137.2, 112.5 and 122.0 and correspond to the 8a, 4b, 4a and 9a positions. The signals at δ 139.4, 115.0, 122.3, 120.2, 128.8 and 112.5 were assigned to other aromatic methine carbons of the B-formula. The 1H and 13C NMR spectra combined with the MS data established the presence of indole system [13]. On reviewing the literature, the spectroscopic data of 8 were found to be identical with these of harmane [13] (Fig. 2).Harmine (4): Compound 4 was obtained as pale white crystals, showed a positive response with the Dragendorff’s reagent. A molecular formula was determined as C13H12N2O from the positive-ion molecular peak at m/z 212. The 1H NMR spectrum of 4 exhibited five aromatic protons at δ 6.90 (1H, dd, J=8.6, 2 Hz H-6), 6.98 (1H, d, J=2, Hz, H-5), 7.72(1H, d, J=5.4Hz, H-3), 7.97 (1H, d, J=6,8Hz, H-5) and 8.33 (1H, d, J=5.4Hz, H-4) of the B formula. In addition, the signals at δ 2.81 (3H, s, -CH3) and 3.81 (3H, s, -OCH3) were attributed to -CH3 and -OCH3 groups, respectively. The -CH3 group i located at C-1 (carbon signal δ 134.9) and the -OCH3 group is located at C-7 (carbon signal δ 160.8). The 13C NMR spectrum exhibited signals of 13 carbons at δ 142.0 (C-1), 137.80 (C-3), 115.0 (C-4), 122.73 (C-5), 109.03 (C-6), 160.8 (C-7), 94.90 (C-8), 141.30 (C-10), 111.20 (C-11), 127.5 (C-12), 134.40 (C-13), 20.4 (-CH3), and 55.43 (-OCH3). These data unequivocally support the molecular structure of the compound 4 as a harmine, described in Figure 2 [14]. Alkaloids genus Peganum well studied, literature data biological activity of determined us alkaloids shown in Table 4.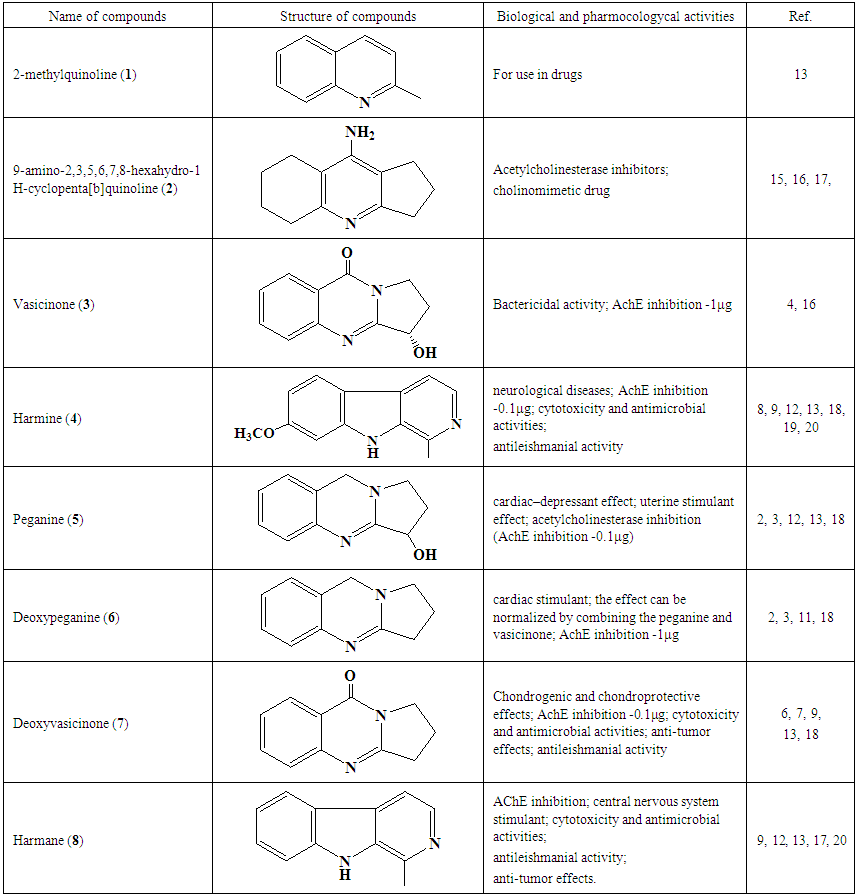 | Table 4. Biological and pharmacological activities of alkaloids found from Peganum multisectum (Maxim) Bobrov |
The following alkaloids have been studied previously, however, as it can be seen from this research, these compounds can be applied for medical purposes. The alkaloid composition of Mongolian P. multisectum, differs little from P. multisectum other regions. The above-mentioned alkaloids have been studied before, however, as can be seen from these studies (Table 4), the compounds can be applied for medical purposes.
4. Conclusions
By the CC and GC-MS, we have determined eight alkaloids from the aerial parts of P. multisectum growing in Mongolia. The alkaloids 2-methylquinoline (1), 9-amino-2, 3, 5, 6, 7, 8-hexahydro-1H-cyclopenta [b] quinoline (2), and harmane were found for the first time from this species. As shown in previous biologicaly studie, the compounds 4, 5, 6, 7 and 8, are active against tumor, bacteria, chondrogenic and chondroprotective effects, leishmania and so on. Therefore, it is important to seek a chance to cultivate this plant and to introduce it in medicinal practice.
References
| [1] | Sheahan M. C., Chase M. W. (1996) A phylogenetic analysis of Zygophyllaceae R. Br. based on morphological anatomical and rbcL DNA sequence data. Bot. J. Linn. Soc., 122, 279-300. |
| [2] | Jinous A., Fereshteh R.P. (2012) Chemistry, pharmacology and medicinal properties of Peganum harmala L. African Journal of Pharmacy and Pharmacology 6(22), 1573-1580. |
| [3] | Nida A., Aijaz A.W., Irshad A.N., et al. (2014) Distribution and Medicinal importance of Peganum harmala-A review International Journal of Advanced Reseach, 2, 751-755. |
| [4] | Grubov V.I. (1982) Opredeliteli sosudistikh rastenii Mongolii, Nauka, Leningradskoe otdelenie, 175. |
| [5] | Narantsetseg A., Shagdar D., Gonchig Tserenbaljid. (2006) Taxonomy of the genus Peganum L (Peganaceae Van Tieghem) In Mongolia. Mongolian Journal of Biological Sciences 4(2), 135. |
| [6] | Cai, Z.L., Liu, Z.B., Duan, J.A et al (1994) Zhonaguo Yaoke Daxue Xuebao 25, 311. |
| [7] | Lamchouri F., Settaf A., Cherrah Y., et al. (1999) Antitumor principles from Peganum harmala. Theraphie, 54, 753-758. |
| [8] | Sobhani A.M., Ebrahimi S.A., Hoormand M., et al. (2009) Cytotoxicity of Peganum harmala L. seeds extract and its relationship with contents of β-carboline alkaloid activity-guided isolation of P. nigellastrum Bunge. Arch. Pharm. Res., 32, 1245-1251. |
| [9] | Zhong Y.C. (2011) Study on chemical constituents of Peganum multisectum. Journal of Chinese Medicinal Materials, 34(11), 1719-21. |
| [10] | Javzan S., Selenge D., Jamyansan Y., et al (2011) Alkaloids from cultivated plant of Peganum harmala L. Mongolian Journal of Chemistry, 12(38), 113-116. |
| [11] | Joshi C.J., Bai Y., Puar M.S., et al (1994) 1H-13C NMR assignments for some Pyrrolo[2, 1b] quinozoline alkaloids of Adhato davasica. Journal of Natural Products, 57, 953-962. |
| [12] | Avula B, Begam S., Ahmed S., et al. (2008). Quantitative determination of vasicine and vasicinone in Adhato davasica by high performance capillary electrophoresis. Pharmazie – An International Journal of Pharmaceutical Sciences, 63(1), 20–22. |
| [13] | Raymond G, Booth., Neal Castagnoli Jr., Hans Rollema et al (1989) Intracerebral microdialysis neurotoxicity studies of quinoline and isoquinoline derivatives related to MPTP/MPP+ Neuroscience Letters, 100, 306-312. |
| [14] | Qi Ch., Rihui Ch., Hongsheng Ch., et al (2005) Antitumor and neurotoxic effects of novel harmine derivatives and structure-activity relationship analysis. International Journal of Cancer, 114, 675–682. |
| [15] | Ishii Y., Sumi T (1992) Evaluation of a cholinomimetic drug, 9-amino-2, 3, 5, 6, 7, 8-hexahydro-1h-cyclopenta [b] quinoline (NIK-247), as an enhancer of endogenous efflux of acetylcholine from brain slices. Neuropharmacology, 31(1), 61-66. |
| [16] | Ono M., Kubota S., Sonoyama W., et al (2013) Novel chondrogenic and chondroprotective effects of the natural compound harmine. Biochimie, 95(2), 374-81. |
| [17] | Louis E.D., Zheng W., Jurewicz E.C., et al (2002). Elevation of blood-carboline alkaloids in essential tremor Neurology. J. Chem., 59, 1940-1944. |
| [18] | Di Giorgio C., Delmas F., Ollivier E., et al (2004) In vitro activity of the beta-carboline alkaloids harmane, harmine, and harmaline toward parasites of the species Leishmani infantum. Experimental Parasitology, 106(3-4), 67-74. |
| [19] | Jahaniani F., Ebrahimi S.A., Rahbar R., et al (2005) Xanthomicrol is the main cytotoxic component of Dracocephalumkotschyii and a potential anti-cancer agent. Phytochemistry, 66(13), 1581–92. |
| [20] | Verdian R., Mohammad R., Hajiakhoondi A (2007) Cytotoxicity and antimicrobial activity of Harmane alkaloids. Journal of Pharmacology and Toxicology, 2(7), 677-680. |








 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML