-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2017; 7(5): 144-149
doi:10.5923/j.cmaterials.20170705.03

Evaluation of in Vitro Bioactivity of 45S5 Bioactive Glass/Poly Lactic Acid Scaffolds Produced by 3D Printing
Santos Adriana Martel Estrada1, Imelda Olivas Armendáriz2, Alecia Torres García2, Juan Francisco Hernández Paz2, Claudia Alejandra Rodríguez González2
1Departamento de Diseño, Universidad Autónoma de Ciudad Juárez, Ciudad Juárez, México
2Departmento de Ciencias Básicas, Universidad Autónoma de Ciudad Juárez, Ciudad Juárez, México
Correspondence to: Imelda Olivas Armendáriz, Departmento de Ciencias Básicas, Universidad Autónoma de Ciudad Juárez, Ciudad Juárez, México.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this study 45S5 Bioactive Glass-based scaffolds produced by 3D Printing techniques have been evaluated. Three-dimensional printing (3DP) technique was used to fabricate scaffolds with a novel micro- and macro-architecture. In this study, Poly Lactic Acid/45S5 bioactive glass composites were produced. 45S5 bioactive glass was synthetized by sol-gel process using microwave exposure. Cylindrical scaffolds of different designs were fabricated. The in vitro bioactivity of the composites was investigated by incubation in simulated body fluid (SBF). The apatite layer was assessed using scanning electronic microscope (SEM) coupled to energy-dispersive electron X-ray spectroscopy and confirmed using X-ray diffraction (XRD) and Fourier-Transformed Infrared spectroscopy (FTIR). Results confirmed that after incubation in simulated body fluid, it was formed a layer of carbonate hydroxyapatite in the surface of the scaffolds.
Keywords: 45S5 Bioactive glass, Poly lactic acid, 3D printing
Cite this paper: Santos Adriana Martel Estrada, Imelda Olivas Armendáriz, Alecia Torres García, Juan Francisco Hernández Paz, Claudia Alejandra Rodríguez González, Evaluation of in Vitro Bioactivity of 45S5 Bioactive Glass/Poly Lactic Acid Scaffolds Produced by 3D Printing, International Journal of Composite Materials, Vol. 7 No. 5, 2017, pp. 144-149. doi: 10.5923/j.cmaterials.20170705.03.
1. Introduction
- In recent years, as an alternative to limitations of current grafting procedures, bone tissue engineering has emerged as a different strategy which provides a solution to regenerate or repair bone tissue [1]. Researchers have taken into account three elements such as osteoinductive growth factors, osteogenic progenitor cells, and osteoconductive biomaterials. The performance of biomaterials (3D scaffold) depends of the success of the material in these aspects (bone tissue engineering). Since these artificial structures act as temporary extracellular matrix, which must allow the adhesion, migration, and proliferation of cells as well as maintain cellular differentiation. In addition, the scaffold must be fabricated with defined morphology (interconnected structure and controlled porosity) as well as suitable mechanical properties [2]. For this reason, it has been used different techniques and multi-phase materials with structure and composition similar to natural bone during the fabrication of the structure.The ability of apatite to form on the surface of a material, is often evaluated in a simulated body fluid [3]. Bioactivity refers to a spontaneous interaction of material with the surrounding biological environment that induce precipitation and mineralization of calcium phosphates on the surface, that it is affected by the physical and chemical properties of it [4]. After the scaffold is implanted into bone defect, it is expected that it well integrate with the surrounding bone without forming fibrous tissue interface layer [5]. Some studies have shown that bioactivity glasses bond with bone more rapidly than other bioceramics [6]. Hydroxyapatite, tri-calcium phosphate (TCP) and bioactive glass have been used widely in biomaterials to interact with tissues and promote osteogenesis. However, the brittle nature of commercial bioactive materials made of HA, results in low mechanical attributes [7].The most common commercial bioactive glass is the 45S5. It is composed of SiO2, Na2O, CaO and P2O5. It contains less than 60% of SiO2, high contents of Na2O and CaO, and a CaO/P2O5 high ratio that make this material bioactive and very reactive in aqueous medias. Bioactive glasses can be synthetized by melting and casting or by sol-gel methods [8-10]. Melting temperatures are above 1100-1300°C. Lower synthesis temperatures, more homogeneous composition and high surface area are the main advantages of sol-gel over the melting and casting process [8, 10-12]. However, the sol-gel technique for synthetizing 45S5 bioactive glass requires several steps that includes: sol and gel forming, aging (usually 24 h at 70°C), drying (Approximately 24 h at 120°C) and a last step for stabilizing and removing the remaining nitrates of the resulting material which is usually performed at approximately 24 h at 700°C. During this last process, crystallization can occurs reducing the glass bioactivity [13]. Microwave processing is another synthesis methodology. It provides a faster, volumetric and more selective heating that results in a reduction of the processing time, energy savings and dimensional stability. Many materials do not absorb the microwaves at low temperatures and therefore additional heating elements are required in the initial stages [14, 15]. In the case of the glasses, it has been reported that they must reach a critic temperature to absorb microwaves [16]. Thus, microwave heating for glass processing is generally used only in some steps of the process (e.g. drying) or using additional heating elements [14, 17].Polylactic acid (PLA) is a linear aliphatic polyesters, which is most used in bone tissue engineering for its mechanical integrity is preserved in vitro or in vivo In addition to being one of the polymers accepted by the Food and Drug Administration (FDA). For being a biodegradable and biocompatible polymer, it has been used in medical applications including tissue engineering [2], surgical suture [18], drug release systems [19], biomedical imaging and detection, solubilization of hydrophobic drugs and bioactive agents [20], dental implant coating [21], fracture fixation [22], among others. Several methods have been used to elaborate scaffolds for tissue engineering such as electrospinning [23], foaming [24], atmospheric pressure plasma assisted modification [25], three dimensional printing [26], supercritical carbon dioxide foaming [27], particle leaching technique [28], freeze drying [29] and so on. 3D printing allows the production of porous scaffold with complex geometries containing internal pore architectures that are reproduced using a computer generated model [30]. Through this method it is possible to produce macropores designed to allow the ingrowth of host tissues [31]. There are more than 40 differents 3D-printing techniques in development, including fused deposition modeling, stereolithography, inkjet printing, selective laser sintering and color jet printing [32]. SBF immersion study is an effective method to evaluate bioactivity by degradation mechanism (weight loss analysis), apatite formation on material surface and calcium to phosphorus ratio (Ca:P) [7].
2. Methods
- Bioactive glass 45S5 was synthetized by sol-gel process (45% SiO2, 24.5% Na2O, 24.5% CaO and 6% P2O5 in molar percentage). Initially, a 1M solution of nitric acid was prepared with H2O, then 33.5 ml of Si (OC2H5)4 (TEOS, alpha Aesar tetraethyl orthosilicate) were added and the mixture was stirred during 60 min to promote hydrolysis. Subsequently, 2.9 ml of (C2H5O)3PO (TEP, Alfa Aesar tetraethyl phosphate), 20.13 g Ca(NO)3 (Alpha Aesar calcium nitrate), 4 ml H2O, and 13.52 g NaNO3 (Alpha Aesar sodium nitrate) were added and stirred during 45 minutes until a clear sol was obtained. The sol was stored in a closed container during 5 days at room temperature to form the gel. Then, the resulting gel was aged in the closed container for 1 day at 70°C and dried at 120°C during 1 day.Finally, for dry gel stabilization, one gram of the dry gel was placed on a mullite crucible isolated with high alumina fiber and exposed to microwave radiation in a conventional household microwave (Emerson, MT3070 model, 120V-60Hz power supply, 2450MHz frequency, 1100 Watts power) during 1.5 to 6 minutes and then quenched in air. Translucid glass was obtained and characterized by a Panalytical X’Pert PRO powder X-Ray diffraction instrument with Cu Kα radiation (λ = 0.15418 nm) at 40 kV and 20 mA, a Fourier-transformed infrared spectrophotometer (Nicolet 6700 FT-IR Thermo Scientific) and a Broker Energy Dispersive X-Ray Spectroscope. For comparative purposes, dry gel samples were also stabilized in a conventional furnace at 700°C during 24 h.PLA/45S5 bioactive glass was fabricated by mechanical mixing. Firstly, PLA powders were obtained using a mill to grind the polymer pellets, Then, PLA were mixed with 45S5 bioactive glass powders mechanically. After this procedure, the mixed was put on an extruder (ExtrusionBot™) to get a filament. For the bioactivity test it was used a sample (1 cm x 1 cm x 0.5 cm) of PLA and PLA/45S5 bioactive glass that was printed previously in a MakerBoot 3D printer using 75 % of filler as a parameter. A previously established method for this test was used [Martel-Estrada, 2011]. Briefly, the samples were immersed in 5 mL of the SBF (pH 7.4) at 37°C. At each time point, the samples were removed from the SBF, rinsed with de-ionized water, and dried at room temperature.After immersion the samples into the SBF for the different periods, the surface morphologies of the deposits were observed using a field emission scanning electron microscopy (Jeol JSM-7000F). The compositions of the deposits were analyzed using an X-ray diffractometer (X Pret PRO PANalytical) and a Fourier-transformed infrared spectrophotometer (Nicolet 6700 FT-IR Thermo Scientific).
3. Results and Discussion
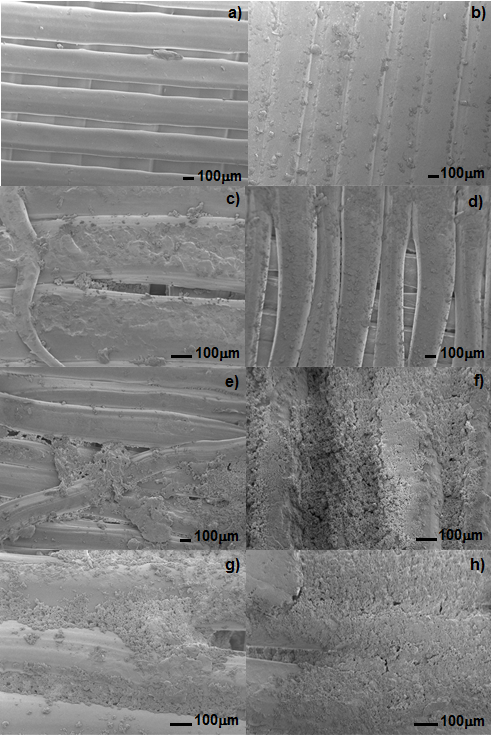
- 3D printing has been employed to fabricate scaffolds for bone engineering to stimulate bone regeneration and combat infection [33]. Poly(L-lactic acid) has been studied for use in biomedical applications such as sutures, scaffolds for tissue engineering, orthopedic devices, or drug delivery systems due to its biocompatibility and bioresorbability [34]. On the other hand, bioactive glass has attracted attention due to its excellent biocompatibility and bioactivity [35]. So, during this research, the bioactivity properties of PLA/45S5 Bioglass composite produced by 3D printing procedure was evaluated. The bioactive glass stabilized by microwave is shown in Figure 1. It was found that the dry gel absorbed the microwaves and no additional heating source is needed.
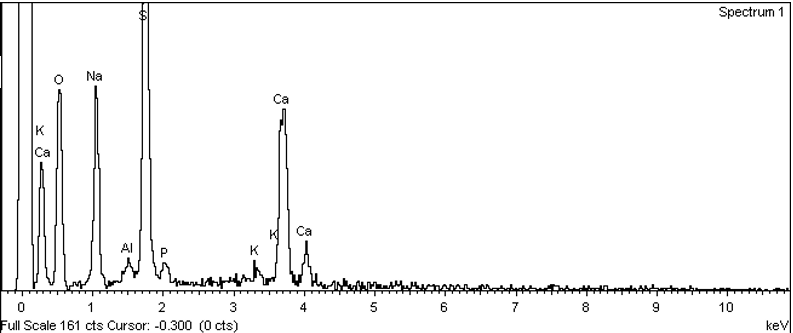 | Figure 3. EDS analysis of the bioactive glass 45S5 |
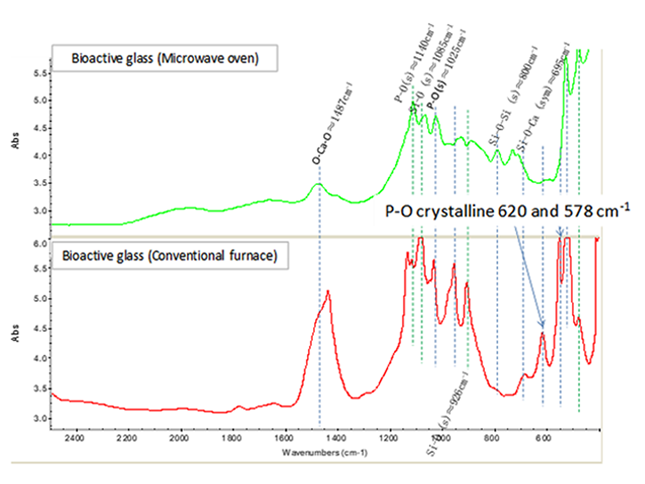 | Figure 4. FTIR analysis comparison of bioactive glass stabilized in conventional oven (700°C, 24 h), and using microwave |
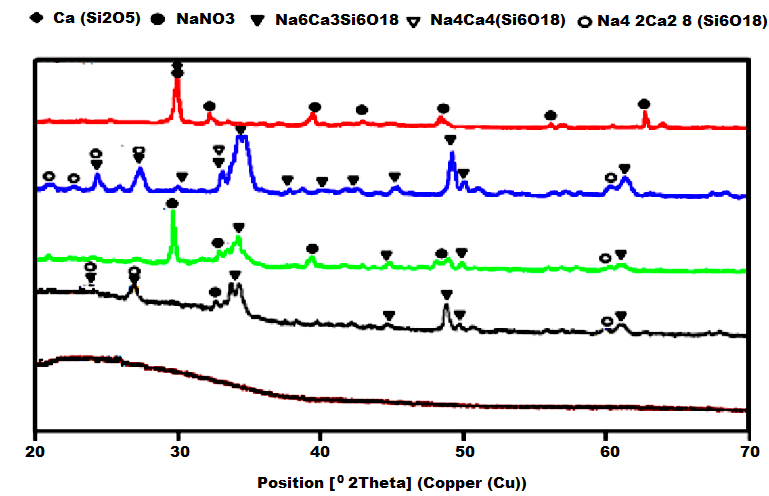
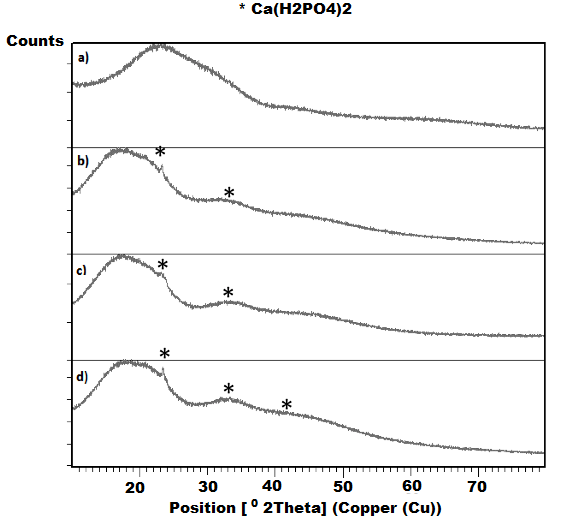 | Figure 6. XRD analysis of PLA scaffolds exposed to the bioactivity test |
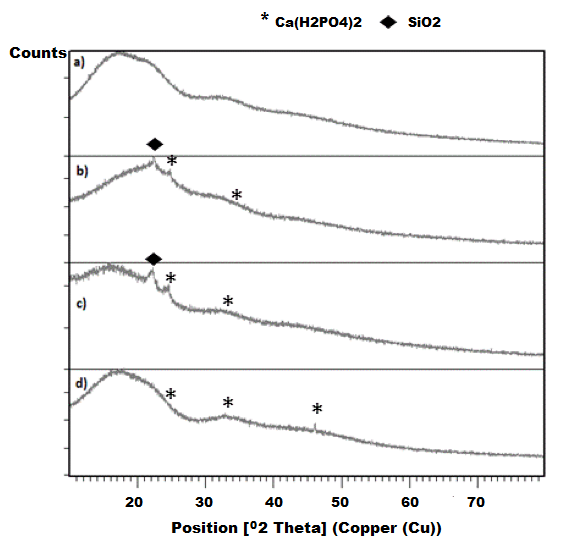 | Figure 7. XRD analysis of PLA/BG scaffolds exposed to the bioactivity test |
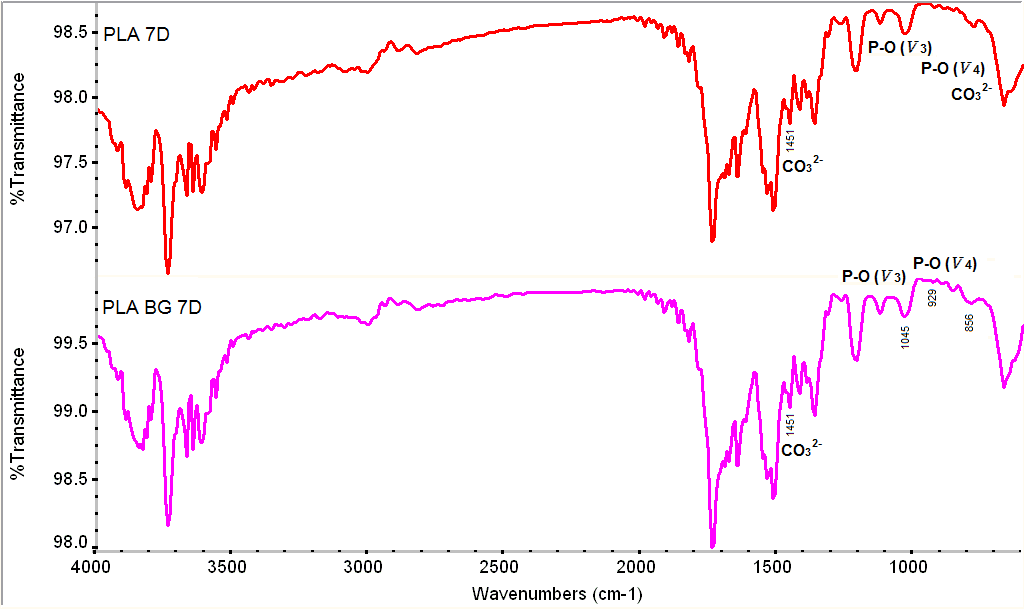 | Figure 8. FTIR spectra for the samples after the SBF treatment |
4. Conclusions
- Bioactive glass 45S5 was successfully synthetized by sol-gel process using microwave for removing nitrates phases from dry gels. The microwave processing allow to reduce the stabilization time (24 h to 7 minutes for X g) and no crystallization occurred maintaining the desired bioactivity. The composition and parameters of printing were selected considering the design of scaffolds with suitable chemical, mechanical and morphological properties for their use in bone regeneration.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML