-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2017; 7(4): 120-126
doi:10.5923/j.cmaterials.20170704.02

Modification of Epoxy Resin with Reactive End-Capped Carboxylic Imide Oligomer for Manufacture of Glass-Fiber Reinforced Composite
Machanje Doreen Itenyo1, Xinhai Yu2, Rotich K. Gideon1
1Department Textile Chemistry, EiTEX, Bahir Dar University, Bahir Dar, Ethiopia
2Department of Applied Chemistry, College of Chemical Engineering and Biotechnology, Donghua University, Shanghai, China
Correspondence to: Rotich K. Gideon, Department Textile Chemistry, EiTEX, Bahir Dar University, Bahir Dar, Ethiopia.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The present work aims at preparing glass-fiber reinforced epoxy resin composite modified with reactive end-capped carboxylic imide oligomer. The carboxylic imide 2,2-bis[4-(4-aminophenoxy)phenyl propane (CIBAPP) oligomer was synthesized via two-step polycondensation of 2,2-bis[4-(4-aminophenoxy)phenyl propane (BAPP) diamine and trimellitic anhydride (TMA), then the oligomer was characterized by FTIR spectrum and solubility test. The oligomer was used as a blending component for the modification of the epoxy resin system for fabricating glass-fiber reinforced laminate. The resultant composite had good properties such as the dielectric strength of 197kV/cm, volume resistance of 2.1×1015Ω•cm, longitudinal and transverse stress of 686MPa and 631MPa, respectively, water absorption rate of 0.18% and surface energy of 43.6mJ/m2.
Keywords: Polyimide oligomer, Epoxy Resin Modification, Composite, Dielectric strength
Cite this paper: Machanje Doreen Itenyo, Xinhai Yu, Rotich K. Gideon, Modification of Epoxy Resin with Reactive End-Capped Carboxylic Imide Oligomer for Manufacture of Glass-Fiber Reinforced Composite, International Journal of Composite Materials, Vol. 7 No. 4, 2017, pp. 120-126. doi: 10.5923/j.cmaterials.20170704.02.
Article Outline
1. Introduction
- Thermosets have been historically the principal matrix material for fiber reinforced composites for many applications. [1, 2] Among the thermosetting polymers, epoxy resins are the most widely used for high performance applications including matrices for fiber reinforced composites, coatings, structural adhesives and other engineering applications [3]. Epoxy resins, apart from them being used with fibers for advanced composite applications, they can also be used in both laminating and molding techniques to make fiber reinforcement with better mechanical strength, chemical resistance and electrical insulating properties [4]. Epoxy based composites are preferred insulating materials for several electrical applications such as printed circuit boards, bushings, generator ground wall insulation system and cast resin transformers [5]. In terms of structural applications requiring high impact and fracture strengths like the aerospace, epoxy resins have limited application as resin matrices. This is because their three dimensional cross-linked network structure renders them brittle and this makes it difficult for them to absorb and distribute stress. Due to this limitation, research has focused on developing ways to toughen the epoxy resins without sacrificing modulus and glass transition temperature (Tg) while retaining their relative low cost, which will in turn lead to an increase in their application [6, 7]. One method which is widely used at present is the incorporation of thermoplastic toughening agents [8-10]. These toughening agents improve ductility of the resin matrix via deformation and cavitation in an otherwise linear, brittle polymer by forming a separate phase from the epoxy matrix. Several approaches can be used to combine the versatility of epoxy resins with the high-temperature properties of imide groups [11-14]. This research has applied the method of blending epoxy resins with thermoplastic polyimides or with functionalized polyimides. This is because this method has been found to be successful for polymeric materials used for industrial applications.In the present work, the multifunctional epoxy resin, modified with end-capped carboxylic imide oligomer (CIBAPP), was applied on a woven glass-fiber to prepare high performance epoxy resin laminate composite.
2. Materials and Experiments
2.1. Materials
- All the chemicals used were analytically pure and were used as received. Toluene, N,N-dimethylformamide (DMF), N-methyl-2-pyrrolidone (NMP), dimethyl sulfoxide (DMSO), tetrahydrofuran (THF), acetone, n n-Dimethylacetamide (DMAc) and aminopropyltriethoxysilane (KH550) silane coupling agent were obtained from Sinopharm Chemical and Reagents Co., Ltd (China); 2,2-bis[4-(4-aminophenoxy)phenyl propane (BAPP) was obtained from Changzhou Pharmaceutical Co., Ltd (China); trimellitic anhydride (TMA) was supplied by Lancaster Synthesis Inc. (U.S.A.); anhydrous ethyl alcohol (abs.EtOH) was obtained from Shanghai Revitalization Chemical Factory (China); 2-ethyl-4-methylimidazole (2E4MI), DER 331 epoxy resin (E51), N,N,N’,N’-tetraglycidyl-4,4’-diaminophenyl methane (TGDDM) and the plain woven glass-fiber(E grade) were all supplied by Shanghai EMST Electronic Material Co., Ltd (China), Carboxyl-terminated butadieneacrylo nitrile (CTBN) was from Lanzhou Petrochemical Research Center (China).
2.2. Synthesis of the End-Capped Carboxylic Imide Oligomer
- In a three-necked round-bottomed flask, 24.63g (0.02mol) of BAPP and 150mL DMAc were added; the mixture was stirred until BAPP was completely dissolved. 23.04g (0.04mol) TMA was then added and stirred until it was completely dissolved. 60mL toluene was then added and the mixture was heated to reflux. The mixture was refluxed at 130-140°C for 3h, and during the reaction time the water was separated. When the reaction was completed, the toluene and 75mL DMAc were removed. The oligomer solution was cooled to room temperature (RT). The synthetic route is shown in Scheme 1. The solid content (ξ) was calculated by use of equation (i):
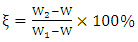 | (1) |
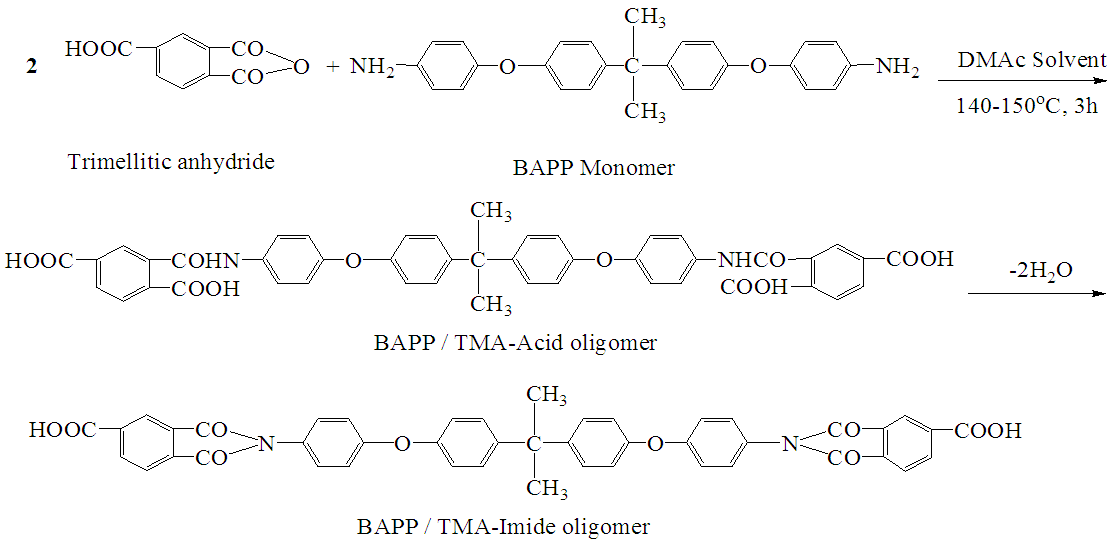 | Scheme 1. Synthetic route of CIBAPP-imide oligomer |
2.3. Preparation the Epoxy Resin Matrix
- 300g epoxy resin (E51: TGDDM=60:40) and 30g CTBN were stirred at 100°C until they were well mixed. The mixture was cooled to below 60°C and 60.1g CIBAPP oligomer solution (20phr) was added, the mixture was stirred at 60°C for 1h, then 4.6g 2E4MI was added while stirring continued at 60°C for 5h to get the epoxy resin matrix. The route is shown in Scheme 2.
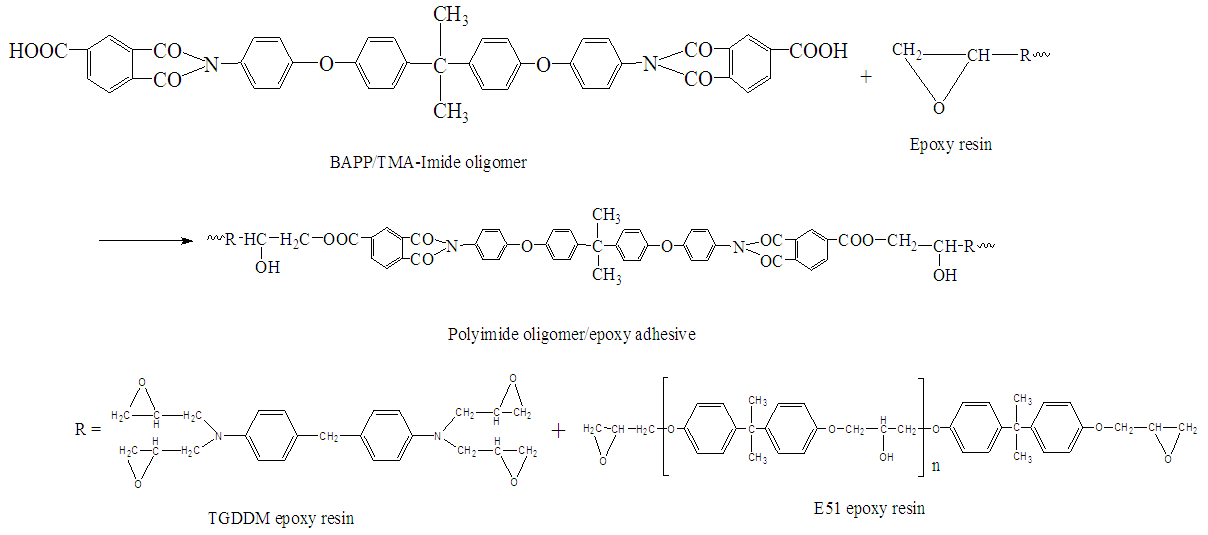 | Scheme 2. Synthetic route for the modified resin matrix preparation |
2.4. Preparation of the Woven Glass Fabric
- The woven glass fabric was cut into 20 equal rectangular shapes with the size of 500 × 300mm and 0.108mm thickness. The total weight of the 20 pieces was 633.12g. Glass fiber has a disadvantage of poor adhesion to polymer matrix resins, therefore to improve their surface adhesion ability; the glass fabric pieces were cleaned by immersing them in a mixture of 3% KH550 silane coupling agent (9mL) and 3L cold water for about 4h. They were left in the oven at 80°C until they were well dried.
2.5. Composite Fabrication
- The modified epoxy resin matrix was uniformly applied on each side of the woven glass fabric, using a metal blade and hanged vertically for overnight drying at RT so that any excess moisture evaporated. The pre-pregs were pre-cured using the following program: RT→40°C /20min→80°C /20min→ 100°C / 20min→RT. The epoxy composite was prepared at 150°C with the pressure of 5MPa for 5mins, before the pressure was raised to 18-20MPa and the temperature to 170°C. It was kept at this temperature and pressure for 4h and cooled to RT under the same pressure to get the composite.
2.6. Measurements
- The FTIR spectrum of the oligomer was scanned on a Varian 640-IR Fourier Transform Infrared spectrometer. The spectral region was 4000~550cm-1. The solubility was done by dissolving the oligomer in various solvents. The gel time test was carried on an ASIDA-NJ11A model gelation times testing instrument manufactured by Zhengye Electronics Company Ltd (China). The viscosity was measured on a CAP2000+cone and plate viscometer model manufactured by Brookfield Engineering Laboratories (U.S.A). The tensile strength test was carried out on a CZ-8000 tensile strength testing machine manufactured by Zhongzhi Instrumentation Company Ltd (China). The contact angle was determined by using XHKE-CATY type instrument from Chengteh Testing Instruments Co. Ltd (China). The Young-Good-Girifalco-Fowkes (YGGF) formula [15, 16] was used to determine the surface energy (γ). The dielectric properties of the glass-fiber reinforced composite were determined as follows: The Composite material’s dielectric breakdown voltage test was done according to GB/T1048.1-2006 standards of China (equivalent to ASTM-D149). The volume resistance test was done according to GB/T 10064-2006 standards of China (equivalent of ASTM-D257). By use of equation (ii), the volume resistance (ohm.cm) was calculated.
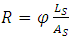 | (2) |
3. Results and Discussions
3.1. CIBAPP Imide Oligomer
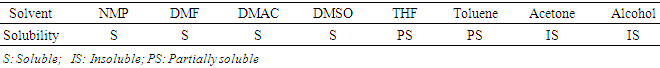
- The CIBAPP oligomer was synthesized via two-step polycondensation to get the transparent dark red sticky solution which gave a light yellow fine powder in water. From Table 1 the powder was readily soluble in polar solvents such as NMP, DMAc, DMF and DMSO, partially soluble in less polar solvent such as THF and toluene and insoluble in alcohol and acetone. This good solubility in polar solvents could be attributed to the ether linkages in the CIBAPP structure which imparted flexibility.
|
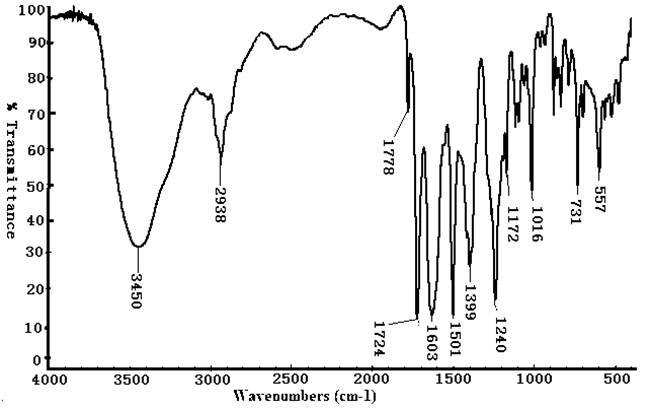 | Figure 1. FTIR spectrum for CIBAPP imide oligomer |
3.2. Characterization of Epoxy Resin Matrix
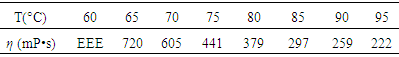
- The viscosity of the epoxy resin matrixThe relationship between viscosity and temperature of the epoxy resin matrix is shown in Table 2. From the table it can be seen that the viscosity decreased with the increase in temperature.
|
|
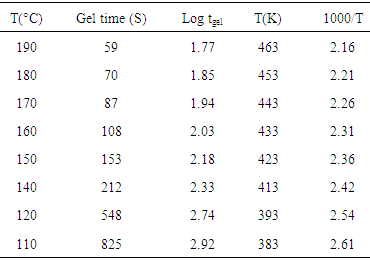
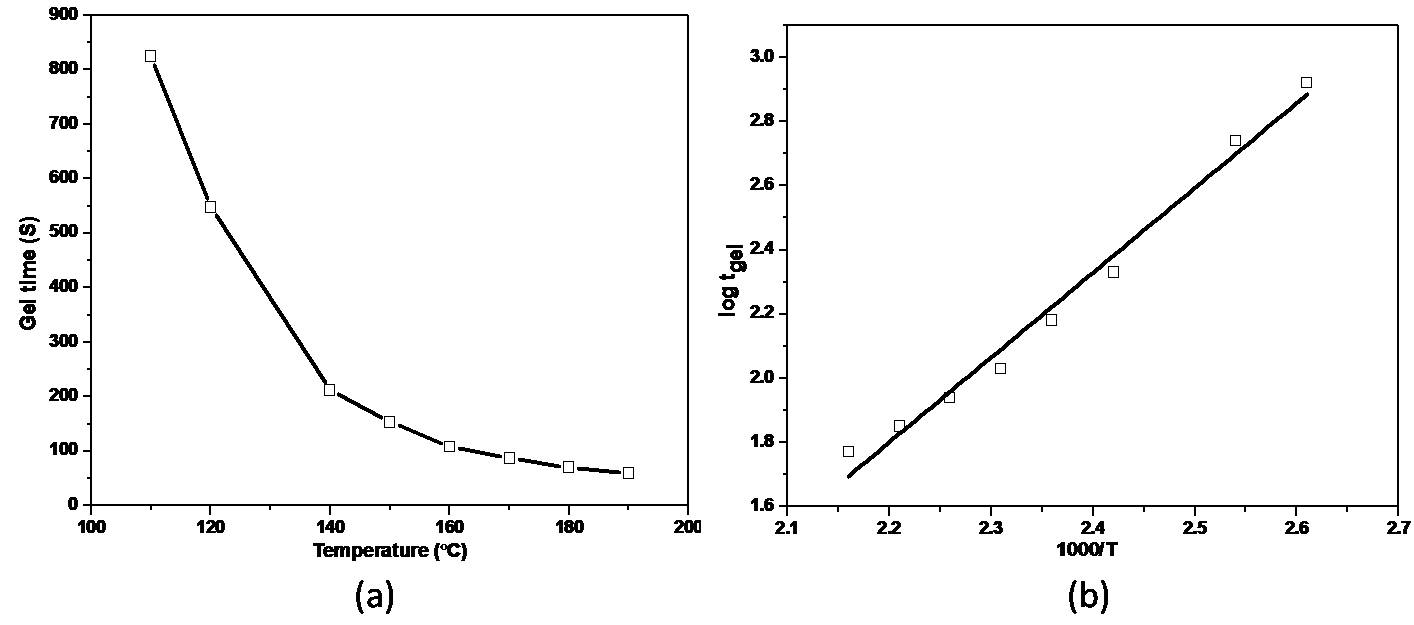 | Figure 2. (a) Gel time verses temperature and (b) log tgel verses 1000-T relationship graphs for the modified resin matrix |
3.3. Characterization of Glass-Fiber Reinforced Epoxy Resin Laminate
- Contact angle and surface energyThe measured values of the contact angles of the glass-fiber reinforced composite of modified epoxy resin sample were as follows: Water 66.6°; glycerine 70.9°, ethyl glycol 48.2° and 1-bromo naphthalene 17.6°. By use of the YGGF equation, the surface energy was calculated and found to be 43.6mJ/m2. This value is less than the surface energy of water (72.8mJ/m2) showing that the composite had good hydrophobicity. Generally epoxy resins are hydrophilic in nature; therefore in this case the modification with the end-capped imide oligomer reduced the hydrophilic nature of the epoxy resin thus increasing its hydrophobicity. Mechanical propertyThe data of the bending test are shown in Figure 3 and the results of the bending test were as follows: longitudinal stress that led to breaking was 686MPa, while the transverse stress that led to breaking was 631MPa. The longitudinal stress was higher than the transverse stress. This is because the sample used was of a plain woven structure. During weaving it is the warp yarns at 0° orientation that are under tension, therefore this tension contributes to the higher loading strength in the glass-fiber reinforced composites because during loading both the fibers and matrix carry the load simultaneously. The lower transverse stress could be attributed to the stitch-induced misalignment to the load bearing tows and the fact that weft yarn have crimp therefore before the fibers carry the load, it has to straighten first. The stress values are higher than the values obtained by Reyes and Sharma [23] indicating that the glass-fiber reinforced composite of modified epoxy resin has stronger mechanical properties.
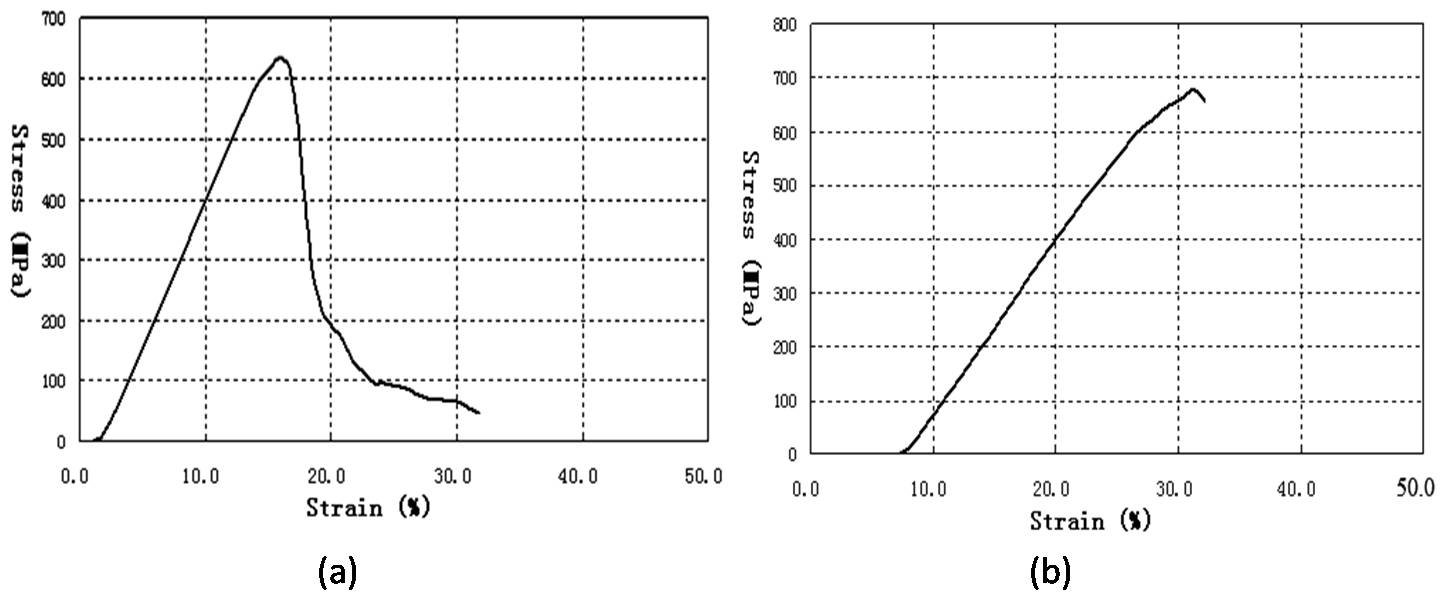 | Figure 3. Three-point bending test results; (a) Transverse and (b) Longitudinal directions |
4. Conclusions
- The modification of a two-component epoxy resin system with an end-capped carboxylic imide oligomer resulted in a resin matrix with excellent mechanical properties, good hydrophobicity and excellent resistance to moisture absorption. When this modified resin matrix was used to fabricate the glass-fiber reinforced laminates, the resultant composite had good insulation capability due to its higher dielectric breakdown voltage (197kV/cm); higher volume resistivity (2.1×1015 Ω•cm) than glass-fiber reinforced composites of nylon and polystyrene; it was also stronger than other fiber reinforced polymer composites due to its high flexural stress values both in the longitudinal and transverse direction. In addition to the above properties, the composite also had good hydrophobicity (43.6mJ/m2), very good resistance to water absorption (0.18%). This composite has better application as an insulating material in microelectronic industries.
ACKNOWLEDGEMENTS
- This work was supported by Fundamental Research Funds for the Central Universities on preparation and property of epoxy matrix (ZX201211000018).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML