-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2016; 6(6): 167-171
doi:10.5923/j.cmaterials.20160606.01

Composite of Triglycidyl para-amino Phenol, Polystyrene and [3-(2-aminoethylamino)propyl] Trimethoxysilane-Modified Graphite
Ayesha Kausar
Nanoscience and Technology Department, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan
Correspondence to: Ayesha Kausar , Nanoscience and Technology Department, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this effort, triglycidyl para-amino phenol (TGAP) epoxy and polystyrene blend was used as matrix material, while 2,4-diaminoanisole (DAN) was employed as hardener. Graphite was first acid functionalized using nitric acid and then chemically modified with [3-(2-aminoethylamino)propyl]trimethoxysilane (AEPTMS). Both the acid functional as well as AEPTMS modified graphite were incorporated as filler in epoxy-based materials. Aim of the study was to see the effect of flame retardancy and mechanical properties of epoxy blend and composite with modified graphite loading. Compressive tests revealed that the compressive failure strength and toughness of 3wt.% silane modified graphite-reinforced epoxy blend were greatly improved relative to acid functional graphite loading and blend. The observation was consistent with scanning electron micrograph of fractured sections, where acid functional graphite sheets were mostly pulled out from the matrix. However, AEPTMS modified graphite depicted fine dispersion due to covalent interaction. Cone calorimetry analysis of Epoxy/PS/GM system exhibited lower heat release rate due to the introduction of AEPTMS modified graphite.
Keywords: Epoxy, Polystyrene, 2,4-diaminoanisole, Toughness, Flame retardancy
Cite this paper: Ayesha Kausar , Composite of Triglycidyl para-amino Phenol, Polystyrene and [3-(2-aminoethylamino)propyl] Trimethoxysilane-Modified Graphite, International Journal of Composite Materials, Vol. 6 No. 6, 2016, pp. 167-171. doi: 10.5923/j.cmaterials.20160606.01.
Article Outline
1. Introduction
- Epoxy resins have been widely employed as matrices in advanced composite materials owing to their excellent mechanical performance, chemical and electrical resistance, and low shrinkage on curing [1, 2]. Compared to other thermosetting polymers, epoxy resins have been largely used in aviation industry due to cost effectiveness. However, neat epoxy resins depict poor non-flammability i.e. a serious limitation in structural applications [3, 4]. So as to encounter flame retardant application requirement, their properties need to be improved without deteriorating other important characteristics such as mechanical and thermal features [5, 6]. Hence, there is a demand for research and development of novel flame retardant epoxy composites. High-performance mechanical and flame retardancy performance at the same time has been a major challenge for aircraft manufacturers [7, 8]. The halogen-free additives have been investigated in this regard keeping in view the environmental concerns [9, 10]. In case of epoxy composites, the effect of reinforcement on solid phase mechanism of flame retardants is still under investigation. One material system of particular interest is epoxy and graphite composites. Epoxy/graphite composite materials have been progressively exploited for numerous space applications [11, 12]. Due to favorable mechanical characteristic of high strength and high stiffness, epoxy/graphite composite have received considerable research interest [13, 14]. Variety of challenges have been addressed using these composite such as size reduction, weight, harsh space environment (radiation and atomic oxygen), longevity, and functionality [15]. Moreover, these materials have been tailored to meet design requirement ranging from slight weight to high structural stiffness. Owing to outstanding performance, epoxy/graphite composites have gained recent research focus [16, 17]. Therefore, this article aims at studying the effect of flame retardancy and mechanical properties of triglycidyl para-amino phenol (TGAP) epoxy and polystyrene blend with modified graphite filler. The role of polystyrene was to decrease the brittleness of epoxy matrix, by increasing the toughness. 2,4-Diaminoanisole (DAN) was used as hardener. Graphite was first acid functionalized using strong nitric acid and then modified with [3-(2-aminoethylamino)propyl] trimethoxysilane (AEPTMS). The modified graphite was then incorporated as filler in epoxy-based materials.
2. Experimental
2.1. Materials
- Graphite (powder, <45μm), [3-(2-aminoethylamino)- propyl]trimethoxysilane (AEPTMS, ≥80%), triglycidyl p-amino phenol (TGAP), 2,4-diaminoanisole (DAN, 99%), tetrahydrofuran (THF, ≥99.9%), and ethanol (anhydrous, ≥99.5%) were obtained from Aldrich.
2.2. Measurement
- Field Emission Scanning Electron Microscopy (FE-SEM) of freeze fractured samples was performed using JSM5910, JEOL Japan. Stress-strain behavior of the samples was studied using Testomeric materials testing machine M500–30CT. The crosshead speed was maintained as 5 mm/min at room temperature. The combustion properties of composite were calculated using cone calorimetry. Samples were tested with FTT 0007 cone calorimeter under heat flux of 50 kW/m2.
2.3. Acid Functionalization of Graphite
- 1 g graphite was refluxed in 100 mL HNO3 at 120°C for 12h. The mixture was poured in 500 mL of deionized water for 24 h. The mixture was filtered and washed several times with deionized water till pH ~7 was obtained. The acid functional graphite was dried in at 60°C for 6 h [18].
2.4. Chemical Modification of Graphite with [3-(2-aminoethylamino)propyl]trimethoxysilane (GM)
- The grafting reaction was carried out in 100 mL of water/ethanol mixture (1:3). A quantity of 3g [3-(2-aminoethylamino)propyl]trimethoxysilane was then introduced. The mixture was refluxed at 80°C for 1 h. Afterward, 1 g graphite was added in the reaction flask. The mixture was further refluxed for 6 h at 80°C. The mixture was filtered and washed several times using water/ethanol mixture. The resultant product was dried at 80°C for 4h [19]. The reaction scheme is shown in Fig. 1.
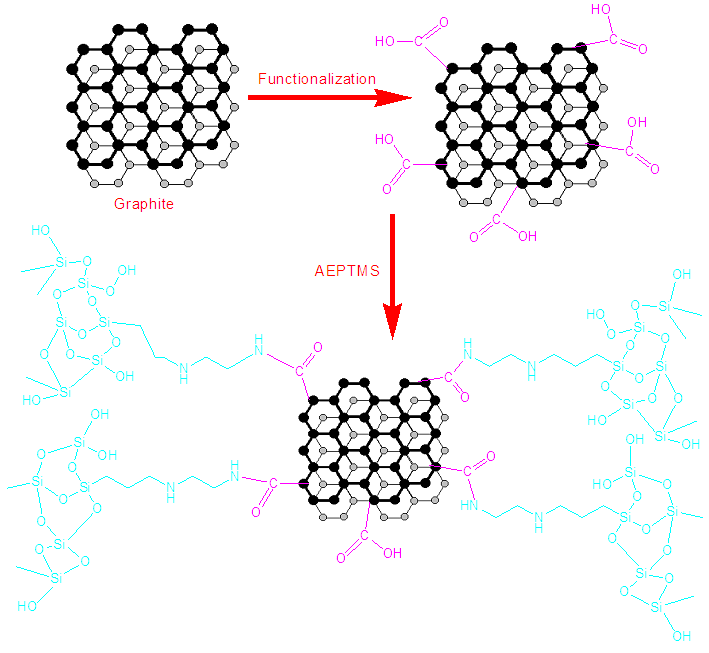 | Figure 1. Scheme for modification of graphite |
2.5. Preparation of Triglycidyl para-amino Phenol/Polystyrene/Modified Graphite (Epoxy/PS/GM) Nanocomposite
- 2g epoxy was dissolved 10 mL THF. 0.1 g PS was separately dissolved in 1 mL THF and added to epoxy mixture. Then, preferred amount of graphite and modified graphite (1-3 wt.%) was added and mixture was refluxed at 80°C. After reflux, the curing agent 2,4-diaminoanisole was added and mixture was again heated for 2h at 60°C. The epoxide to amine ratio was adjusted as 1:1 [20].
2.6. Preparation of Triglycidyl para-amino Phenol/Polystyrene/Graphite (Epoxy/PS/G) Nanocomposite
- The composite was prepared using the same procedure mentioned in Section 2.5. Except that the graphite added was acid functional and not the AEPTMS modified.
3. Results and Discussion
3.1. Mechanical Study
- Fig. 2 shows the stress-strain curves of neat blend, Epoxy/PS/G 3, and Epoxy/PS/GM 3. The composites showed a clear change in compressive behavior relative to blend. The failure strength showed a marked increase compared with the neat blend in compressive failure strength as well as toughness. Toughness was measured by integrating area under stress-strain curves. In Epoxy/PS/GM 3, the compressive strength was increased by 63%, while toughness by 67%, relative to neat blend.
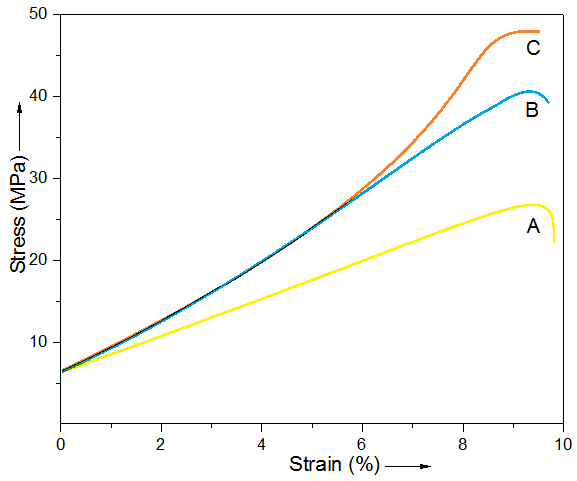 | Figure 2. Stress-strain curves of (A) neat blend; (B) Epoxy/PS/G 3; and (C) Epoxy/PS/GM 3 |
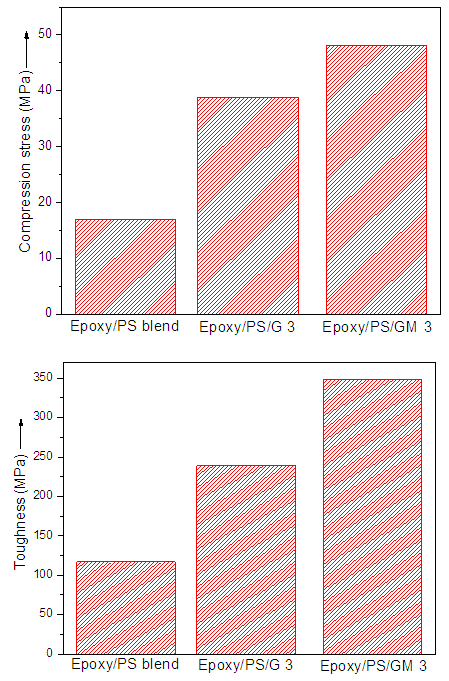 | Figure 3. Compression stress and toughness curves of (A) neat blend; (B) Epoxy/PS/G 3; and (C) Epoxy/PS/GM 3 |
3.2. Morphology Analysis
- To get more information about the interfacial interaction between the functional filler sheets, fractured sections after compressive tests were investigated by FESEM. As shown in Fig. 4, most acid functional graphite sheets were observed to be pulled out from the epoxy/PS matrix (Fig. 4A). Due to non-uniform dispersibility of acid functional graphite sheets, the filler pull out was observed. On the other hand, most of AEPTMS functionalized graphite sheet were embedded in the epoxy/PS matrix (Fig. 4B). No functional sheets were observed to be pulled out, indicating that the graphite sheets had stronger covalent interfacial bonding with the matrix. Therefore, the fracture did not occur at the sheets/interface. Hence, due to uniform dispersibility of modified sheets and strong interfacial linkage between the filler and matrix, high load-transfer efficiency was obtained (Fig. 4C). Consequently, the mechanical properties of the system were greatly improved. Generally, high strength and high toughness are difficult to achieve at same time, however modified graphite-reinforced composite with strong interfacial linkages do demonstrate this exceptional property as well [23-25].
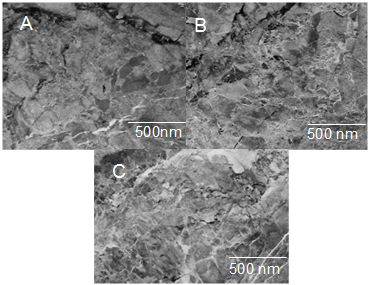 | Figure 4. FESEM images of (A) neat blend; (B) Epoxy/PS/G 3; and (C) Epoxy/PS/GM 3 |
3.3. Flammability Test
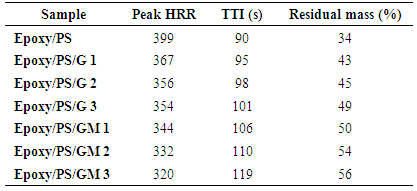
- According to the mass loss type cone calorimeter results, the neat blend showed lower flame resistance. Comparing the flame retardancy of the blend and composite in Table 1 shows that the Epoxy/PS/GM system exhibited lower heat release rate (HRR). The peak heat release rate (PHRR) was decreased significantly due to the introduction of AEPTMS modified graphite. The time to ignition (TTI) was increased in case of Epoxy/PS/GM composite. Comparing the amount of residues after combustion, the introduction of 3 wt.% AEPTMS modified graphite (GM) led to an additional increase in residual mass. The residual mass of Epoxy/PS/GM 3 composite was 13% higher than the Epoxy/PS/G 3, while 39% higher than the neat epoxy blend. The novel composite showed enhanced non-flammability compared with carbon nanotube, graphene, and POSS reinforced epoxy composite [26-30].
|
4. Conclusions
- In summary, triglycidyl para-amino phenol (TGAP) epoxy and polystyrene blend was successfully formed with diaminoanisole hardener. Graphite was linked with 3-(2-aminoethylamino)-propyl]trimethoxysilane via covalent functionalization. Afterwards, reaction between AEPTMS functionalized graphite sheet and epoxy groups also occurred. The sheets of modified graphite were well dispersed in Epoxy/PS matrix. Furthermore, silane modified sheets were introduced as reinforcing component via covalent functionalization for the first time. Owing to the homogeneous dispersion in matrix and covalent bonding between AEPTMS functionalized graphite sheet and epoxy groups, the compressive failure strength and toughness was improved significantly compared to the neat blend and graphite loaded system.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML