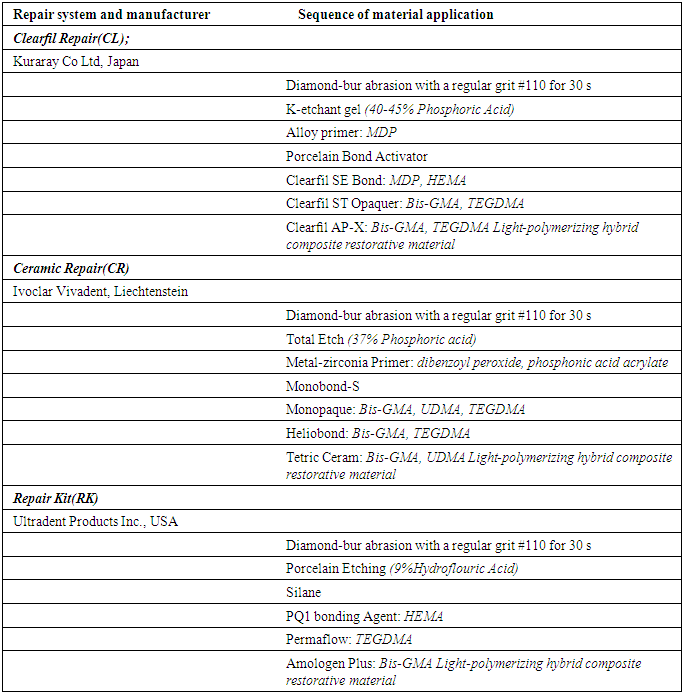
-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2016; 6(4): 121-128
doi:10.5923/j.cmaterials.20160604.04

Comparison of the Shear Bond Strength of Three Different Composite Materials to Metal and Ceramic Surfaces
Gulsum Sayin Ozel1, Ozgur Inan2
1School of Dentistry, Department of Prosthodontics, Istanbul Medipol University, Istanbul, Turkey
2Faculty of Dentistry, Department of Prosthodontics, Selcuk University, Konya, Turkey
Correspondence to: Gulsum Sayin Ozel, School of Dentistry, Department of Prosthodontics, Istanbul Medipol University, Istanbul, Turkey.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Fracture of metal ceramic restorations can be repaired intraorally to postpone or eliminate the remake. Many different ceramic repair materials are available; the bond strength is the most important data for predicting the success of the repair systems. This study evaluated the shear bond strength of three different repair systems for metal-ceramic and full-ceramic restorations applied on metal, zirconia and veneering porcelain. Substrates to which porcelain repair materials would be applied were prepared in a 10-mm diameter and 3mm thickness. Thirty cylindrical specimens (9 x 3 mm) were fabricated in a nickel-chromium alloy (Kera-N) and thirty in feldspathic porcelain (Ceramco). Metal (M) and porcelain (P) specimens were embedded in a polyvinyl chloride (PVC) mold and after completing the preparation of the surface, three different composite resins (n=10): Clearfil Ceramic Repair System/Clearfil AP-X (Kuraray), Ceramic Repair System/Tetric Ceram (Ivoclar Vivadent), Ceramic Repair System/Amolegen Plus (Ultradent) in a 4 mm diameter and 2 mm thickness were applied to the central region of the specimens. The specimens were stored in distilled water for 24 hours at 37°C, thermal cycled (1000 cycles at 5°C to 55°C), and stored at 37°C for 8 days. Shear bond tests between the metal or ceramic specimens and repair systems were performed in a mechanical testing machine with a crosshead speed of 0.5 mm/min.The data were analyzed by a one-way ANOVA. Ceramic Repair System/Clearfil AP-X (Kuraray), showed significantly highest mean shear bond strength value for metal-ceramic substrates (P = 0.00).
Keywords: Shear-bond strength, Adhesion of repair sets, Metal-Ceramic repair systems
Cite this paper: Gulsum Sayin Ozel, Ozgur Inan, Comparison of the Shear Bond Strength of Three Different Composite Materials to Metal and Ceramic Surfaces, International Journal of Composite Materials, Vol. 6 No. 4, 2016, pp. 121-128. doi: 10.5923/j.cmaterials.20160604.04.
1. Introduction
- Despite the fact that prosthetic use of full ceramic materials have developed and grown up, metal ceramic restorations have a large clinical usage in dentistry because of their mechanical strength [1, 2]. Nonetheless, the mostly seen clinical problem at metal-ceramic restorations is structure of fragile ceramic veneer. In the literature, porcelain fracture originated failures have been reported to be occurred at a rate of 2.3-8% [3, 4]. Fractures in general have been occurred due to several reasons such as, trauma, incompitable occlusal arrangements, parafunctional habits, flexural fatigue of metal infrastructure, incompatible thermal expansion coefficient between metal infrastructure and porcelain, insufficient dental preparation, porosities in the structure of porcelain, metal infrastructure design [5, 6]. Three kinds of fractures have usually been monitored at metal ceramic restorations: simple fractures (formed only within porcelain and metal does not get out of surface), mixed fractures (as well as porcelain fractures, metal gets out of surface), complex fractures (metal completely gets out of surface) [6, 7].Porcelain fractures are the most common cause of removing the prosthesis. Fractured porcelain affects patients negatively in terms of aesthetic and function and requires to be changed [6, 8]. In this case, two different treatment options come to mind. The primary and ideal treatment option involves removing the prosthesis that not always applicable and financially costly. An alternative method is repair of fractured area with composite resin intraorally [9, 10]. Intraoral repair method offers some advantages such as, economic cost and time savings. But, the bond between restoration remained in the repaired area and repair material should be strong and resistant to the functional loads [11]. In order to improve the bond between composite and fractured surfaces, many mechanical and chemical bond methods have been developed. To provide the mechanical bond; many surface treatments including roughening with diamond drills, sandblasting with aluminium oxide have been used for both metal and ceramic surfaces. Hydrofluoric acid roughening, acidylophosphat fluoride or ammonium hydrogen bifluoride have been used for ceramic surfaces roughening. Regarding chemical bonding, adhesive primers and silane coating agents can be applied after mechanical surface treatments in order to strengthen bonding [6, 10, 12].In addition, companies have intended to strengthen the bond between composite resin and metal ceramic surfaces by various primary and bond systems included in repair sets in themselves through developing adhesive systems [12]. It has been intended to improve existing repair systems to exclude use of surface treatment application procedure and loss of time. The aim of this study is to evaluate and compare the adhesive bonds of three different porcelain repair systems with two surfaces such as, feldspathic porcelain and nickel-chromium base metal alloy without any surface treatment. The null hypothesis was there is no difference of shear bond strength between the repair systems both for porcelain and metal substrates.
2. Material and Method
- A total of 30 cylindrical samples in 10 mm diameter and 3 mm thick, were prepared from nickel-chrome metal alloy and feldspathic porcelain (Ceramco3; Dentsply, Germany). The samples of every substrate were randomly divided into three groups and three different repair systems were applied to each group (n = 10).During the preparation of metal samples (M), modelling of wax (S-U-Gnatho-Wax. Schuler Dental, Germany) was prepared from polyvinyl chloride (PVC) mold in 10 mm diameter and 3 mm thick (Fig. 1). The modelling of waxes prepared were placed in silicone casting cuffs and then embedded in phosphate bonded investment (Bellavest SH; Bego, USA). After pouring of investment has been completed in a twenty minute period, silicone cuff removed and investment was placed into an oven heated to 800°C (MFX-1010 Cuff Furnace, Microtek Dental, Turkey). The oven was heated up to 950°C so that modelling of wax could melt and kept in the oven at 950°C for 30 minutes in compliance with manufacturer's instructions. After completion of burn-out, casting process was performed at induction casting device (Inf-2010; Microtek Dental, Turkey). The resulting metal samples were removed from investment and sandblasted with 100μm aluminium oxide powders to be cleaned from investment (MKK.975S 2; Microtek Dental, Turkey). Similar PVC molds were used during the preparation of porcelain samples (P) as well. Porcelain powder and liquids were mixed in the proportions in line with the manufacturer’s instructions and injected into the molds and placed in the porcelain oven preheated at 600°C (Multimat C; Dentsply, Germany). It was heated up 930°C and was left to cool to ambient temperature.
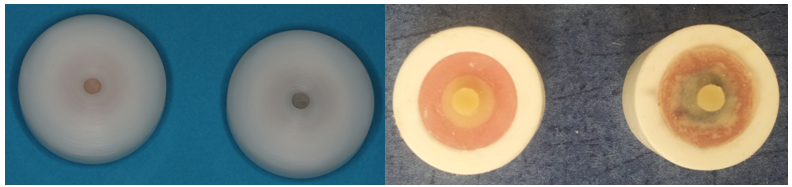 | Figure 1. PVC matrix used for application resin opaque and resin composite |
 | Figure 2. Shear bond strength testing apparatus |
 | Figure 3. Repair kits applied metal and porcelain substrates |
|
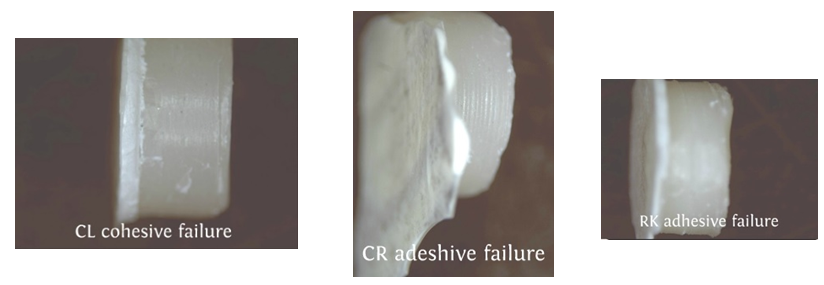 | Figure 4. View of the failure type for metal substrates |
 | Figure 5. View of the failure type for porcelain substrates |
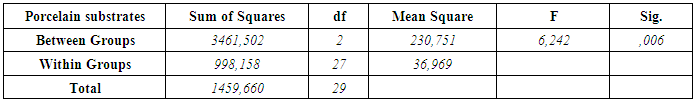
3. Results
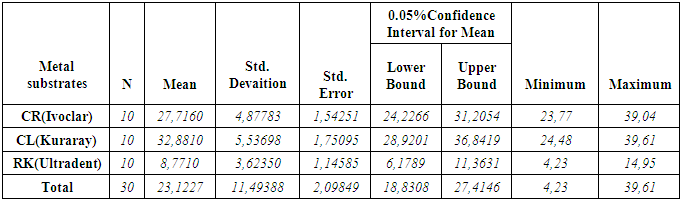
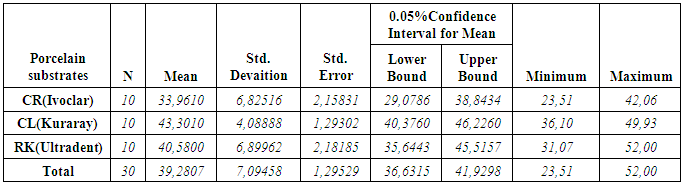
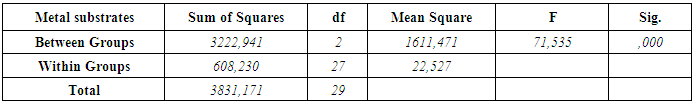
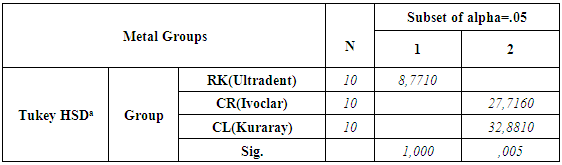
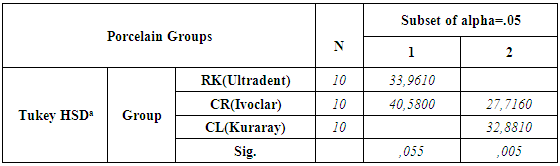
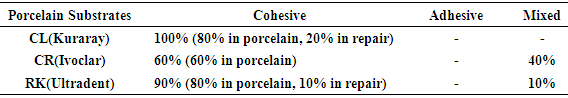
- According to the One-Sample Kolmogorov-Smirnov Test we can understand that the values show normal distrubution hence the data were analyzed by a parametric test One-Way ANOVA. As a result of the statistical analysis made, it was determined that there was a significant difference between metal groups p=0.000 and porcelain groups p=0.006 (p<0,05). Regarding metal substrates, the maximum value was identified in Kuraray CL group, the minimum value was identified in Ultradent RK group. Regarding Porcelain groups, the maximum value was identified in Kuraray CL group, the minimum value was identified in Ivoclar Vivadent CR group. The average bond strength values of groups and their standard deviations were given in Table 2a-Table 2b and the analysis results of one-way Anova test and binary comparison Tukey HSD test were given in Table 3a-3b and Table 4a-4b. In respect to microscopic evaluation of the failure types; as well as Kuraray CL group revealed cohesive failure, 60% in opaque and 40% adhesive failure for metal substrates; on the other hand, both ivoclar vivadent CR and ultradent RK groups showed 100% failure. In respect to porcelain substrates; as well as Kurary CL group revealed 100% cohesive failure, 80% in porcelain and 20% in repair resin; Ultradent RK group revealed 90% cohesive failure, 80% in porcelain and 10% in repair resin, and 10% mixed failure. Ivoclar Vivadent CR group revealed cohesive failure, 60% in porcelain and 40% mixed failure (Table 5a, Fig. 4.-Table 5b, Fig. 5).
|
|
|
|
|
|
|
|
4. Discussion
- The aim of this study was to compare and evaluate the shear bond strength of three different repair sets without applying extra surface roughening treatment to porcelain and metal surfaces.Regarding porcelain fractures of fixed restorations; restoration replacement, which is ideal treatment approach, can be postponed for some reasons such as, increasing costs, difficulty of removing restoration and trauma formation risk at abutment teeth while removing and waste of time [9]. Therefore, provided that fractured restorations protect periodontal health and do not necessitate replacing, then restoration repair can be considered [13]. The primary objective in restoration repair is to ensure recovering of function and aesthetics of restoration using various repair materials [6, 10]. The most important factor for this purpose is strength of bond between repair materials and restoration. For the purpose of ensuring of strength, various surface treatments or different featured porcelain repair kits have been developed [14]. In the literature, there are many studies, in which, different surface treatment applications for many different surfaces such as, metal porcelain zirconia and repair sets bond strength were evaluated and compared [6, 8]. It is difficult to determine the most appropriate way in repair process due to some reasons such as, multitude of studies, difficulty and cost of applied surface operations, taking time of application procedures [15, 17]. In addition, application of these surface treatments may be difficult in clinical conditions for repairing procedures. For this goal, there have been kits manufacturers claimed through which, they could increase the bond between porcelain or metal surfaces and repair composite without the need for extra surface roughening treatment. Bailey and Bennet reported as a result of their studies that use of hybrid composite resulted in better bong strength than composites of microphile filler in respect to porcelain repair restorations [18]. Also in this study, the same manufacturer’s proposed hybrid composites were used for each repair kit. In addition, for the evaluation of bond’s strength, shear bond strength test was applied in the study. That’s why porcelain repair process is necessary especially for buccal area of the restorations and anterior region restorations were usually associated with shear forces. Apart from that, the most commonly used test in the literature in terms of evaluation of porcelain repair system for different substrates is shear connection test [19].According to the results, all bond strength values between porcelain and composite resulted in stronger bond than between metal and composite in all repair systems. The null hypothesis was rejected. This can be explained with the effect of acid roughening and silane agents on porcelain surfaces. Kupiec et al. revealed that application of hydrofluoric acid, especially using it with silane agents increased bond strength. Similarly, Stangel et al. reported in their study that roughening with hydrofluoric acid increases the bond strength between porcelain and composite [20, 21, 22]. While the adhesion between the metal surfaces and resin could only be succeeded with opaque and opaque enhancive primers; acid agents and silane agents included in sets have a great effect on porcelain, which increases the adhesion between porcelain and composite [12].With respect to metal surfaces, while Chung and Hwang [15] reported that sandblasting with aluminium oxide causes the bonding between composite and metal structure alloys to increase, Suliman et al. reported that there was not any significant difference between sandblasting, diamond bur roughening and application of hydrofluoric acid [16]. In line with these studies, due to the fact that roughening treatments with diamonds for metal and porcelain surfaces is a method aimed to simulate clinical conditions and often used in repair operations inside mouth, it was applied to each sample without exception. Since alloy primers and composites containing diphosphate monomer (MDP) have phosphate ester groups, they can directly form a bond with metal oxides and superior bond strengths were evaluated for metal alloys [23]. Kuraray Clearfil repair (CL) group showed the highest metal-composite resin bond strength. This situation may be resulted from the effect of alloy primer and MDP content of Clearfil SE bond. It was reported that adhesive materials containing MDP revealed great bonding properties for base metal alloys [24, 25]. Similarly, the result of our study suggested that Kuraray CL system revealed the greatest bond for metal substrates in terms of opaque bond. This bond increase can be explained with MDP content. Accordingly, failure types regarding Kuraray CL systems were mostly identified as cohesive failure. In line with these studies, cohesive failure was observed in Kuraray CL group for metal substrates as a result of the study. Depending on the increase of the bond strength between opaque and metallic substrate through alloy primer, cohesive failure (cohesive failure 60% in opaque - 40% adhesive failure) instead of adhesive type failure was observed. The group with lowest composite reinforced shear bond strength is ultradent repair kit (RK) group for metal substrates. This failure can be explained with that hydrofluoric acid does not have as much effect as porcelain on metal, and the bond between metal and opaque remain weaker. The reason why adhesive failure type is mostly seen in RK group can be that the bond between metal substrate and opaque is weaker. Regarding feldspatic porcelains, acid etching constitutes micromechanics undercuts having a decisive effect for a better bond. It was reported in many studies that combined use of micromechanics roughening and silane agents constituted a strong bond for porcelain surfaces. Silane agents are dual functional monomers. It consists of cylanol and methacrylate groups. While cylanol group reacts with ceramic surface, methacrylate group becomes copolymerized with resin matrix of composite material [24, 26, 27]. It has been known that silane coupling agents help glass ceramic substrates’ wettability to increase with resin composites and resin composites help mechanical and chemical bond to increase with resin composites [28]. In addition, some authors proposed use of hydrofluoric acid for repair of silica contained ceramics since hydrofluoric acid causes silicium dioxide (SiO2), which is glass phase of ceramics, to form a microporous structure by affecting it and also allows constitution of a mechanical interlock formation between composite resin and ceramic [29]. Ultradent RK group suggested high bond values in the study because of that hydrofluoric acid produced good roughening and wettability on porcelain and additionally, positive effect of silane agents on the bond in respect to groups of porcelain. Due to the fact that Kuraray CL groups and Ultradent RK groups revealed more effective porcelain roughening than 37% orthophosphoric acid of Ivoclar CR group because of having respectively 40% thixotropic phosphoric acid and 9% hydrofluoric acid contents, they offered greater bond strength than Ivoclar CR group among porcelain groups. In line with these results, Ozcan reported that 37% orthophosphoric acid can be considered to offer lower efficiency [10]. The greatest bond strength was revealed by Kuraray CL group among porcelain groups. This situation could be explained by that silane agents effect of porcelain bond activator and clearfil SE bond primer made the adhesion more stronger. Kuraray CL groups increased the bond positively with MDP content inside them for both porcelain and metal groups.16-20 MPa of bond strength was reported as acceptable value clinically especially for bond strengths [30]. In many different study, shear strength values reported for various porcelain repair kits are between the values of 6 and 29.9 MPa [31]. In our study; regarding all porcelain and metal substrates; when shear bond strength values of three different porcelain repair kits and their restorations were compared; while the values in all groups were above the acceptable values and utilizable clinically, Ultradent RK group’s values suggested for metal substrates were lower than the clinically acceptable values and in this case, use of metal surface roughening treatments might be more useful in use of ultradent repair kit clinically for metallic surfaces.
5. Conclusions
- Shear bond strength of the composite materials to the metal and porcelain surfaces is important for clinical practice of metal-ceramic repair systems. In line with limitations of this study; Kuraray CL groups revealed highest bond strength resulted from MDP content and 40% thixotropic acid efficiency in both metal and porcelain substrates. The bond strength of Ultradent RK group is the lowest among metal groups. During using this repair kit on metal surfaces, additional surface roughening treatments may be more useful for clinical application.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML