-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2015; 5(6): 195-201
doi:10.5923/j.cmaterials.20150506.08

X-ray Studies and Electrical Properties of the Zinc-substituted Copper Nanoferrite Synthesized by Sol-gel Method
Sabah M. Ali Ridha
Department of Physics, College of Education for Pure Sciences, University of Kirkuk, Kirkuk, Iraq
Correspondence to: Sabah M. Ali Ridha , Department of Physics, College of Education for Pure Sciences, University of Kirkuk, Kirkuk, Iraq.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The structural and dielectric properties were studied for Zn substituted copper spinel ferrite having the formula Cu1-xZnxFe2O4 where [x = 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0]. This ferrite prepared by an auto-combustion sol-gel method and calcined at different temperatures (500, 800, and 1100°C). The X-ray diffraction pattern of these compositions showed the formation of the single phase spinel structure. Furthermore, the XRD have been used to calculate the lattice parameter, particle size, bulk density and porosity for all ferrite samples. The particle size varied from 18.5 nm to 36 nm, while the lattice parameter decreased with calcination temperatures and Zn-ion content in the range (8.2688-8.3304 A°). X-ray density decreases from 5.536 to 5.562 g/cm3 and porosity increases from 6.4 to 12.8% with increasing Zn-ion content. The dielectric constant decreases from 56*103 to 26*103 and the loss tangent from 8.52 to 0.33 with frequency (in the range of 50 Hz – 5MHz), while the dielectric constant takes high values with high Copper content ferrites. a.c. Conductivity increases (from 0.27*10-5 to 168 *10-5 Ω-1.cm-1) with increasing frequency. On other hand; A.C. Resistivity decreases (from 361*103 to 0.59*103 Ω.cm) with frequency for all Cu-Zn ferrite samples.
Keywords: Cu-Zn ferrites, ac. conductivity, Spinel ferrites, Nanoferrite samples, Auto-Combustion method
Cite this paper: Sabah M. Ali Ridha , X-ray Studies and Electrical Properties of the Zinc-substituted Copper Nanoferrite Synthesized by Sol-gel Method, International Journal of Composite Materials, Vol. 5 No. 6, 2015, pp. 195-201. doi: 10.5923/j.cmaterials.20150506.08.
Article Outline
1. Introduction
- Spinel structure ferrites are an important class of electrical materials due to their high electrical resistance and low eddy current losses which make them useful in radiofrequency circuits, high quality factors, transformer core, magnetic recording media, magnetic fluids and other devices [1-5]. Polycrystalline nanoferrites are very good dielectric materials and depends on several factors, including the method of preparation, sintering conditions, chemical composition, crystallite size and cation distribution in the two sublattices i.e. tetrahedral (A) and octahedral [B] lattice sites, [6-17]. According to the crystal structure, copper ferrite is an inverse spinel ferrite and Zn ferrite is normal spinal, and the substitution of zinc in copper ferrite modifies the dielectric properties of copper ferrite which are useful in many device applications [18, 19]. Cu-Zn nanoferrite play significant role among ferrite materials due to their high electrical resistivity, high dielectric and low tangent losses [20, 21]. Hence it is important to study their dielectric behavior at different frequencies. The auto-combustion sol-gel method has been chosen in the present study to synthesize the Cu-Zn nanoferrites (Cu1-xZnxFe2O4; where x= 0.0 to 1.0) at temperature 220°C, and calcined at temperatures 500, 800, 1100°C. Spinel structures, crystallite size, lattice parameter, and theoretical densities were characterized using X-ray diffractometry patterns date for Cu-Zn ferrites. The variations of dielectric constant, dielectric loss and a.c. conductivity for Cu-Zn ferrite samples sintered at 1100°C with different Zn content have been studied as a function of frequency using impedance analyzer LCR meter in the range 50Hz- 5MHz.
2. Experimental
2.1. Synthesis
- Cu-Zn ferrites of composition Cu1-xZnxFe2O4 (where; x=0-1.0) were synthesized using auto-combustion sol-gel method. The appropriate amount of nitrates [Zn(NO3)2.6H2O, Cu(NO3)2.3H2O and Fe(NO3)3.9H2O] and citric acid [C6H8O7..H2O] were dissolved in distilled water to form aqueous solution. The pH value of solution was adjusted to pH=7 using ammonia solution. Then the aqueous solution was heated and evaporated at 90°C under intensive stirring to transform into a highly viscous gel. The obtained gel is dried inside electric oven at a temperature of 120°C. Auto combustion reaction is initiated by heating the dry gel at 220°C.The as-burnt powders were divided into four parts and calcined separately at temperatures 220, 500, 800, and 1100°C for 3h] and then mixed with small amount (2% weight) of saturated polyvinyl-alcohol aqueous solution and uniaxially pressed at 3 ton/cm2 pressure to form pellet shaped samples with diameter ~12 mm and thickness ~1.5 mm. The pellet samples were sintered at 1100°C for 3 hours. High purity silver paint was added to the each face of the samples for good electric contacts.
2.2. Characterization
- Structural characteristics of ferrite samples were determined by X-ray diffraction patterns obtained in the (2θ) range of (20°) to (70°) with (Cu Kα) radiation (λ =1.5406 Å). The average particle size was determined by using Scherrer formula assuming all the particles to be in spherical in shape, [22].
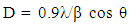 | (1) |
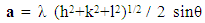 | (2) |
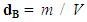 | (3) |
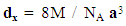 | (4) |
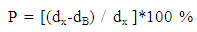 | (5) |
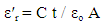 | (6) |
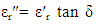 | (7) |
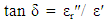 | (8) |
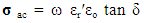 | (9) |
3. Results and Discussions
3.1. Structural Properties
3.1.1. XRD Characteristics of 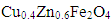
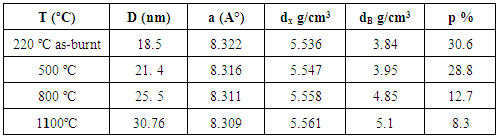
- XRD studies have been carried out on the dried gel (120°C), the as-burnt (220°C) powder, and the ferrite samples calcined at 500, 800 and 1100°C. Figure (1) shows the XRD patterns of five Cu.0.4Zn0.6Fe2O4 ferrite powders. The dried gel powder (at 120°C) is amorphous in nature. The as-burnt powder (at 220°C) is a single phase Cu-Zn ferrite with spinel structure; similar to that of the as-calcined samples at temperatures 500, 800, and 1100°C. The observed peaks at (220), (311), (222), (400), (422), (511), and (440), confirmed the spinel structure of the samples. This indicates that the Cu-Zn ferrite can be directly formed after the auto-combustion of the gel without calcinations.
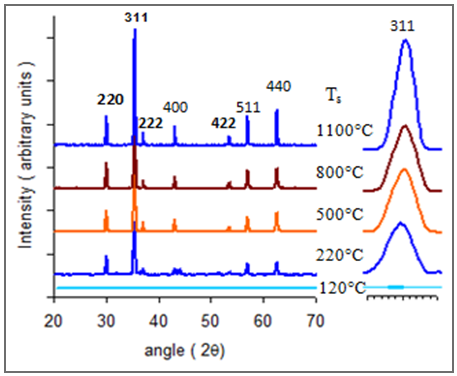 | Figure (1). XRD patterns of Cu0.4Zn0.6Fe2O4 ferrite calcined at different temperatures |
|
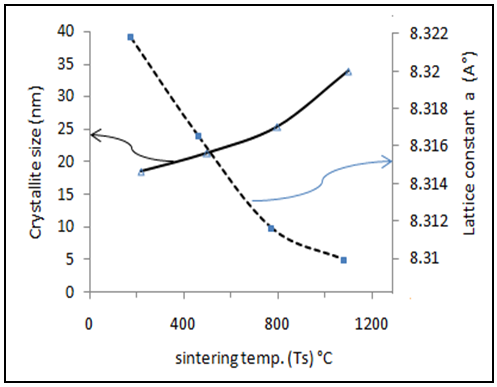 | Figure (2). Variation of crystallite size and lattice parameter of the Cu0.4Zn0.6Fe2O4 ferrite with calcinations temperatures |
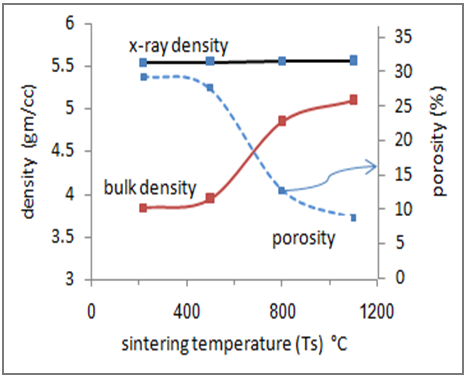 | Figure (3). Variation of x-ray density, bulk density and porosity of the Cu0.4Zn0.6Fe2O4 ferrite with calcinations temperatures |
3.1.2. XRD Characteristics of Cu1-xZnxFe2O4
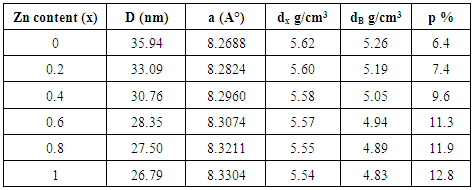
- The X-Ray powder diffraction pattern for the Cu1-xZnxFe2O4 ferrite samples (where; x=0.0, 0.2, 0.4, 0.6, 0.8 and 1.0) sintered at 1100°C for 3 h are shown in figure (4). The observed peaks (220), (311), (222), (400), (422), (511), and (440) confirmed the spinel structure of the samples.
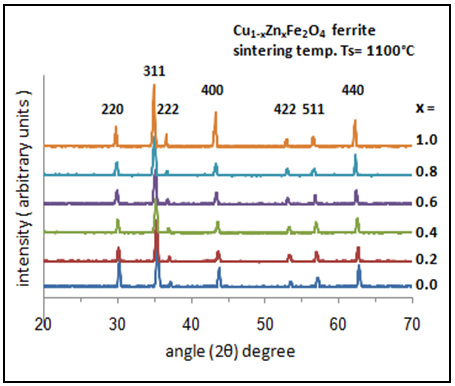 | Figure (4). XRD patterns of Cu1-xZnxFe2O4 ferrite samples calcined at 1100°C with different x value |
|
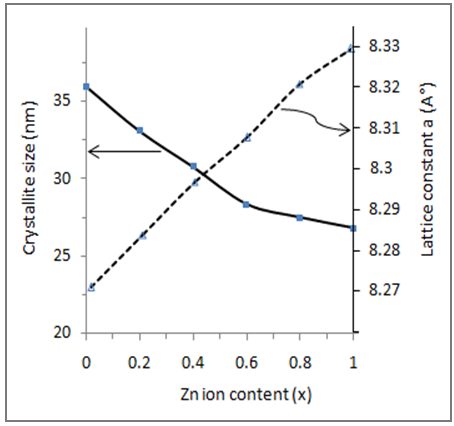 | Figure (5). Crystallite size and lattice parameter of Cu-Zn ferrite samples with respect to Zn content |
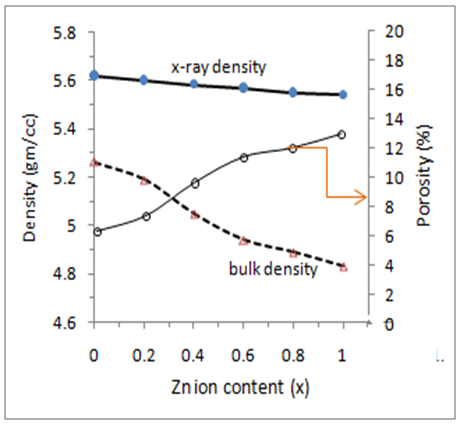 | Figure (6). x-ray density, bulk density and porosity of Cu-Zn ferrite samples with respect to Zn-ion content |
3.2. Dielectric Characteristics
- The variation of dielectric constant, dielectric loss tangent and a.c. conductivity with Zn-ion content were studied as a function of frequencies in the range of (50Hz to 5MHz) as shown in figures (7-14). It is seen that all the parameters decrease with increasing frequency and Zn-ion content.
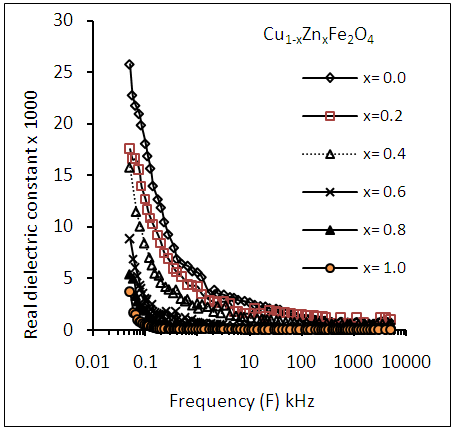 | Figure (7). Variation of dielectric constant (εr' ) as a function of frequency for Cu-Zn ferrite samples for different Zn-ion content (x) |
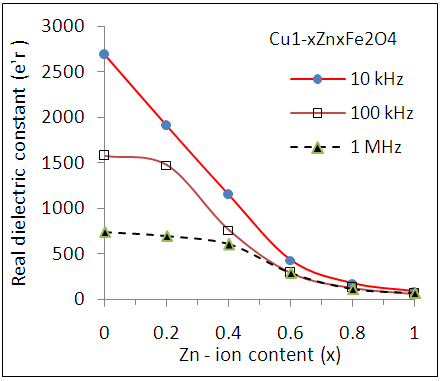 | Figure (8). Variation of dielectric Constant (εr') as a function of Zn content (x) for Cu-Zn nanoferrite samples at different frequencies |
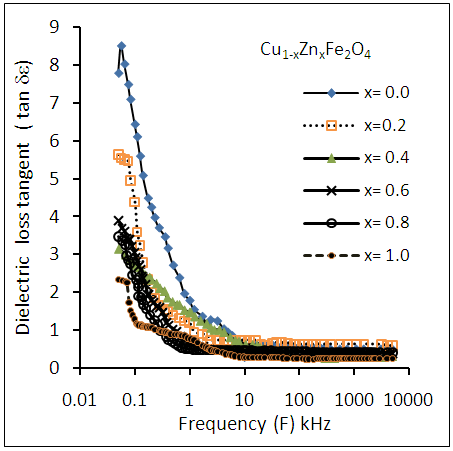 | Figure (9). Variation of dielectric loss tangent (tan δε) as a function of frequency for Cu-Zn nanoferrite samples with different Zn content (x) |
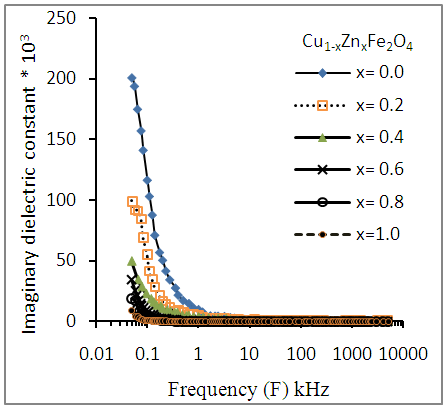 | Figure (10). Variation of dielectric loss (εr") as a function of frequency for Cu-Zn nanoferrite samples with different Zn content (x) |
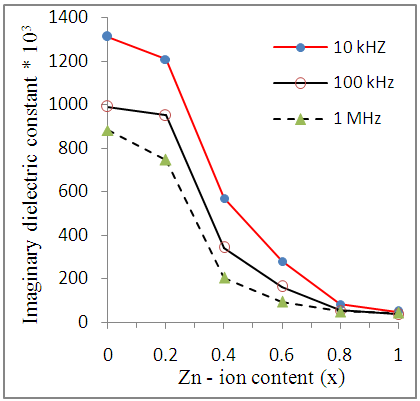 | Figure (11). Variation of dielectric loss (εr") as a function of Zn-ion content (x) for Cu-Zn nanoferrite samples at different frequencies |
3.3. ac. Conductivity
- The a.c. conductivity (σa.c.) of Cu-Zn ferrites has been carried out at different frequencies in the range (50Hz to 5MHz) with different Zn-ion content (x). It was observed from the figure (12) that the σac increases with increasing applied frequency, while it decreases with increasing Zn-ion content (x) at each frequency.
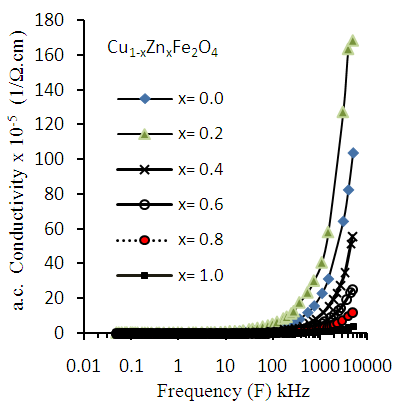 | Figure (12). Variation of a.c. conductivity (σa.c.) as a function of frequency for Cu-Zn nanoferrite samples with different Zn content (x) |
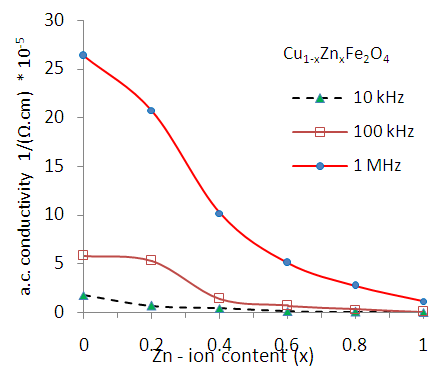 | Figure (13). Variation of a.c. conductivity (σa.c.) as a function of Zn content (x) for Cu-Zn nanoferrite samples with different frequencies |
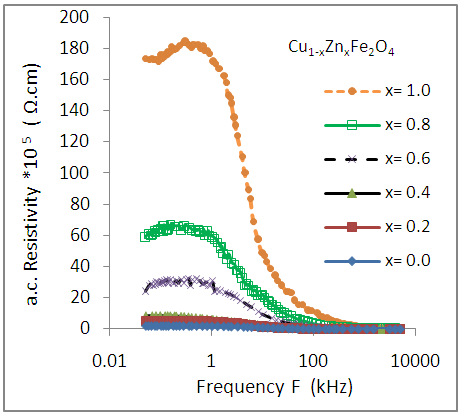 | Figure (14). Variation of a.c. resistivity (ρa.c.) as a function of frequency for Cu-Zn nanoferrite samples with different Zn content (x) |
4. Conclusions
- The auto combustion technique yields nano crystalline single phase Cu-Zn ferrites. The XRD pattern shows the formation of single phase cubic spinel structure for all the samples. Lattice constant and porosity increase whereas X-ray density and bulk density decreases with increasing Zn-ion content in Cu-Zn samples. The crystallite size of the ferrite compositions varies from 27nm to 36 nm. Dielectric constant decreases with the increase in Zn-ion content. Dielectric constant and dielectric loss tangent both decrease with the increasing frequency and can be explained on the basis of space charge polarization. a.c. conductivity increases with increasing frequency, while, it decreases with increasing Zn-ion content This is due to the good conductivity of copper than that of Zinc. On the other hand, the a.c resistivity decreases with increasing frequency, while increases with increasing zinc content .The substitution of Zinc ion in the Cu1-xZnxFe2O4 ferrites causes appreciable changes in its structural, dielectric and electric properties. The obtained experimental results provide important information on improving properties of Cu- ferrites for electronic application.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML