-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2015; 5(6): 167-176
doi:10.5923/j.cmaterials.20150506.05

Biocomposites Scaffolds for Bone Tissue Engineering
I. Olivas-Armendáriz1, E. Santos-Rodríguez2, M. L. Alvarado-Gutiérrez3, Z. A. Meléndez-Molina3, L. A. Márquez-Chávez1, L. E. Valencia-Gómez1, C. L. Vargas-Requena3, S. A. Martel-Estrada4
1Instituto de Ingeniería y Tecnología, Universidad Autónoma de Ciudad Juárez, UACJ. Ave. del Charro 450 Norte, Cd. Juárez, Chih. México
2MCTP/UNACH, Ciudad Universitaria Carretera Emiliano Zapata Km. 4, Real del Bosque (Terán). Tuxtla Gutiérrez, Chiapas, México
3Instituto de Ciencias Biomédicas, Universidad Autónoma de Ciudad Juárez, Henry Dunant 4016, Zona Pronaf, Cd. Juárez, Chihuahua, México
4Instituto de Arquitectura, Diseño y Arte, Universidad Autónoma de Ciudad Juárez, UACJ, Ave. del Charro 450 Norte, Cd. Juárez, Chih. México
Correspondence to: S. A. Martel-Estrada, Instituto de Arquitectura, Diseño y Arte, Universidad Autónoma de Ciudad Juárez, UACJ, Ave. del Charro 450 Norte, Cd. Juárez, Chih. México.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Chitosan/Extract of Mimosa tenuiflora composite scaffolds were fabricated by freeze-drying lyophilization and were then evaluated and compared for use as a bone regeneration scaffold through the evaluation of its bioactivity and biocompatibility. The in vitro bioactivity evaluation of the scaffolds was carried out by analyzing the apatite layers produced on them using simulated body fluid (SBF) as an incubation medium. The apatite formation was analyzed using FTIR spectroscopy and Field Emission Scanning Electron Microscopy coupled with energy-dispersive electron X-ray spectroscopy. The cumulative results obtained from IR spectra and SEM-EDS suggest that the developed composites might have potential applications in tissue engineering. The in vitro cell culture of Wistar rat’s osteoblasts were used to evaluate the phenotype expression of cells in the scaffolds, characterizing the cellular adhesion, proliferation, and alkaline phosphatase activity. Our results, thus, show that Ch/Extracts of M. tenuiflora scaffolds are suitable for biological applications.
Keywords: Chitosan, Mimosa Tenuiflora, Biocompatibility, Bioactivity
Cite this paper: I. Olivas-Armendáriz, E. Santos-Rodríguez, M. L. Alvarado-Gutiérrez, Z. A. Meléndez-Molina, L. A. Márquez-Chávez, L. E. Valencia-Gómez, C. L. Vargas-Requena, S. A. Martel-Estrada, Biocomposites Scaffolds for Bone Tissue Engineering, International Journal of Composite Materials, Vol. 5 No. 6, 2015, pp. 167-176. doi: 10.5923/j.cmaterials.20150506.05.
Article Outline
1. Introduction
- The scaffold material that mimics the structure and mechanical properties of the natural bone is an interesting topic in bone tissue engineering. These scaffolds should have a highly porous matrix for the transportation of nutrients, oxygen and metabolic products [1]. In addition, the chemical composition and surface chemistry play an important role in promoting proliferation and differentiation of cells, including the apatite-forming bioactivity for potential use in bone [2]. The mineralization of materials is carried out in a solution containing ions with type and concentrations similar to those existing in the human body, that are called simulated body fluid (SBF) [3].Polymeric scaffolds and their composites are commonly used in tissue engineering. Polymer-based composites with bioactive ceramic particles were used to improve osteoconductivity and bioactivity [4]. On the other hand, natural products are considered as a source of molecules for the effective treatment of diseases [5]. The medicinal importance of plants is due to the presence of flavonoids, alkaloids, terpenoids, tannins, steroids [6], polyphenols, and polysaccharides that act specifically on the relevant cells [7].Chitosan (Ch) is a natural biopolymer in the biomedical area due to their excellent biological properties, such as biocompatibility, biodegradability, and nontoxic properties [8]. Functional groups, such as carboxyl and hydroxyl groups, facilitate the entrapment of biological particles such as antibiotics, growth factors, proteins, and bone forming cells [9]. Mimosa tenuiflora (M. tenuiflora) (Willd.) Poiret is a plant native of Northeast Brazil and Southeast Mexico and is popularly known as “jurema-preta” and “tepezcohuite,” respectively [10]. The plant is rich in tannins [11], alkaloids, lipids, lupeol, kukulkanins, saponins, and arabinogalactans [12]. The powdered bark has been traditionally used to treat skin burns and wounds and prevent inflammation [12]. Previous studies performed with M. tenuiflora confirmed that fibroblast reacts with a strong improvement of viability and proliferation in the presence of arabinogalactan polymers from the plant [12] and also can stimulate monocytes to release TNF-α [13].Arabinogalactans-proteins (AGPs) are glycosylated proteins of the hydroxyl-rich glycoprotein family. It has been reported that these proteins contain 90–99% of carbohydrate and 1–10% of amino acid. The carbohydrate moiety of AGPs is rich in galactose and arabinose. The AGPs are a water soluble polysaccharide [14] that are highly biocompatible [15].With these characteristics, it could be reasonable to believe in the bioactivity and biocompatibility of a Chitosan/Extract of M. tenuiflora. Therefore, in this study, different biodegradable and biocompatible Chitosan/Extract of M. Tenuiflora composites were manufactured through thermally induced phase separation method. These composites were used to investigate the in vitro biodegradation and in vitro bioactivity of scaffolds by analyzing the apatite layers produced in them using SBF as an incubation medium. The apatite formation was analyzed using Fourier Transform Infrared spectroscopy, X-ray Diffraction, Field Emission Scanning Electron Microscopy (SEM), and Energy Dispersive Spectrometry. Moreover, in vitro cell culture of Wistar rat osteoblasts was used to evaluate the phenotype expression of cells in the scaffolds, characterizing the cellular adhesion, proliferation and alkaline phosphatase activity.
2. Materials and Methods
2.1. Materials
- Chitosan was purchased from Sigma (United States). The bark of M. tenuiflora was obtained from the region of Jiquipilas, Chiapas. Glacial acetic acid (Mallinckrodt, United States) was used as the solvent. SBF was prepared in our laboratory as a previously published method [16].
2.2. Characterization of Metabolites from Extract of Mimosa Tenuiflora
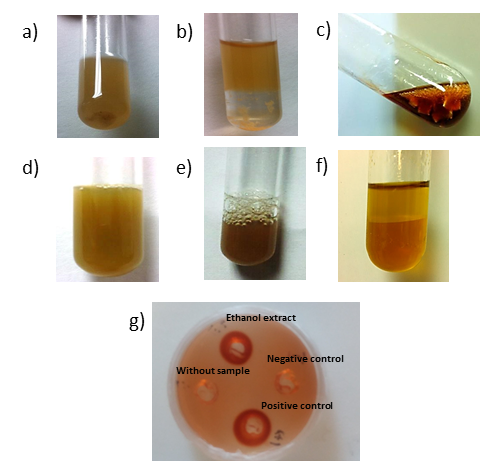
- An amount of 0.5 ml of the M. tenuiflora extract of was collected and tested with the addition of a few drops of Mayer’s reagent to obtain the cream color precipitation for confirming the presence of alkaloids [17]. To evaluate the presence of steroids and triterpenoids, the Liebermann - Burchard reaction was used [6]. In addition, the extract was also examined for the presence of saponins using 4 ml of the extract and shaking for 2 minutes until it formed foam, which was stable for 15 min or more. Then 1% FeCl2 was used in water for phenols detection and gelatin-salt test for tannins [18]. For the flavonoid test, 4 ml of the extract was used, and 3 ml of ethanol was added to it. Then magnesium powder and a few drops of concentrated HCl were added [18]. For the quinones test, 1 ml of H2SO4 was added to 3 ml of the extract. The mixture was heated to evaporation point to produce the hydrolysis. Then 5 ml of toluene was added. About 2 ml of this organic phase was taken and then 1 ml of 5% NaOH in 2% ammonia was added to it [19]. The presence of an AGP was confirmed using a qualitative test by Yariv reagent (ᵝ-D-Glucosyl Yariv Reagent, Biosupplies). A radial agar diffusion test [13] was slightly modified and used to measure the AGP content in the arabinogalactans solution. Briefly, agarose gel (1%) containing 0. 15 M of sodium chloride, 0.02% sodium azide and 0.002% of Yariv reagent was poured into an 80 mm Petri dish. The gel was allowed to dry at room temperature, and then wells were punched. A solution gum Arabic (0.01 g/ml) and distilled water were used as a positive and negative control, respectively. Petri dishes were incubated in the dark at room temperature for 2–4 days until a precipitin halo developed.
2.3. Preparation of Composites
- Ethanol precipitated extract of M. tenuiflora was obtained by a method similar to the one proposed by Zippel [12]. Briefly, 50 g of powdered bark from M. tenuiflora was mixed with 200 mL of distilled water and mixed for 3 days under strong stirring. After every 20 h, the solution was filtered by gravity three times. Then the extract was filtered, concentrated in a vacuum oven at 37°C. Then the solution was resuspended in 200 mL of water, and the extract was precipitated into 800 mL of ice-cold ethanol (96%). The resultant precipitate was isolated by centrifugation for 10 min (3000 rpm), dissolved in 15 mL of water, and dialyzed with cellulose membranes (MWCO 3.5 kDa). The resultant of the dialyzed procedure was yield to 15 mL with distilled water. This solution is the arabinogalactans solution, which was used for the composite preparation. Chitosan/extract of M. tenuiflora composites were prepared in our laboratory according to the procedure described below. Our goal was to prepare compositions that contain exactly the quantity of metabolites contained in 80/20 and 70/30 composites of Chitosan/Bark of M. tenuiflora in weight. Two types of composites were prepared. The 80/20 composite corresponded to 0.300 mL of ethanol extract solution by each 4 g of chitosan. The 70/30 composite corresponded to 0.450 mL of ethanol extract solution by each 3.5 g of chitosan. The ethanol extract solution and chitosan were dissolved in 1% (%v/v) aqueous acetic acid solution. Finally, the composites were frozen and freeze-dried for 2 days.
2.4. Characterization of Morphology and in vitro Bioactivity
- For the in vitro bioactivity study, porous samples of approximately 1 cm × 0.5 cm × 0.5 cm were soaked in 5 mL of tetraethoxyl orthosilicate for 2 h in a vacuum oven at 60 °C. Then the samples were rinsed with ethanol and dried in still air at room temperature for 24 h. Afterward, the samples were soaked in 5 mL of 1.5X SBF, pH 7.4 for different periods of time in an incubator at 37 °C. The solution was refreshed every 48 h. After the incubation period, the samples were rinsed with deionized water and dried.In order to characterize the morphology, a Field Emission SEM JEOL JSM-7000F coupled with an energy-dispersive system EDS 7557 INCA Oxford Instruments (England) were used. The Ca/P ratio was estimated for the various conditions. The pore size was measured using the Scandium Universal SEM Imaging Platform software. Three different cross-sections of each scaffold were used with at least 120 pores. The porosity was determined by a liquid displacement method used previously [8].
2.5. FTIR and XRD Spectroscopy
- The chemical characterization was evaluated in FTIR. FTIR spectra were recorded using a transmission mode in an IR spectrometer (Nicolet 6700, Thermo Scientific, USA). For each spectrum, 100 scans at 16 cm-1 resolution were averaged.
2.6. In vitro Degradation
- Chitosan/extract of M. tenuiflora scaffolds were immersed in PBS (pH 7.4, containing NaN3). Scaffolds of uniform dimensions approximately 1 cm × 0.5 cm × 0.5 cm 3 mm) were used to perform a degradation test. Samples were collected after predetermined time periods (1, 3, 7, 10 and 14 days), washed with DI water, and dried at room temperature for 2 days. The remaining weight of the scaffolds was determined using the following equation (n = 3):Weight loss (%) = [(wi−wd)/wi × 100]Where wi is the initial weight of the scaffold and wd is the dry weight of the scaffolds after incubation.
2.7. Cell Harvesting and Culture
- About 2 weeks old Sprague-Dawley rats were used for the isolation of osteoblasts from calvarian. The rat calvaria tissues were dissected and dissociated [20]. The scaffolds were sterilized by exposition to UV light for 2 h and then osteoblasts calvaria cells were pooled into the scaffolds at a density of 50,000 cells/cm2 and cultured in the prepared solution of α-MEM supplemented with 10% fetal bovine, 1% antibiotics, 2.1 mg/ml β-glycerophosphate, and 50 µg/ml ascorbic acid in an incubator at 37°C with 5% CO2. Cultures were finished at 24 and 72 h.
2.8. Scanning Electron Microscopy (SEM) Examination of Cell-seeded Scaffolds
- After being cultured for different times, the samples with attached cells were rinsed twice with PBS (pH 7.4) and immersed in PBS containing 3% glutaraldehyde for 4 h to fix the cells, followed by rinsing twice with PBS for 10 min and finally dehydrated through a series of graded ethanol solutions. A field emission SEM JEOL JSM-7000F coupled with an energy-dispersive system EDS 7557 INCA Oxford Instruments (England) were used to characterize them.
2.9. Biochemical and Differentiation Analyses
- Scaffolds with osteoblasts calvaria cells with a density of 50,000 cells/cm2 were used as a culture test. The cells were cultured in the prepared solution of α-MEM supplemented with fetal bovine, antibiotics, β-glycerophosphate and ascorbic acid in an incubator at 37°C with 5%. Control and test cultures were harvested at 24 and 72 h and washed twice with PBS. The osteoblasts were lysed with 3 ml of 1% Triton X-100 in DEPC-treated water and three freeze-thaw cycles at −70°C. The alkaline phosphatase activity in the lysed cells was determined using an alkaline phosphatase substrate assay kit and the absorbance measured at 405 nm.
2.10. Cell Viability and Proliferation
- The cell viability was determined using a previous method [21]. Briefly,3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used. At each cultured time, MTT solution (50 μL) and a-MEM (450 μL) containing FBS and antibiotic were added to each composite. After 3 h of incubation, 400 μL of dimethyl sulfoxide (DMSO) was added to dissolve the formazan product, and the absorbance was measured at 570 nm. The cell number was determined with a standard curve. The analysis of cell was evaluated using a Quant- iTTM PicoGreen dsDNA kit. The fluorescence emission intensity was measured at 520 nm. The amount of DNA was determined based on the standard curve.
2.11. Statistical Analysis
- The data obtained were evaluated for statistical significance using the Student’s t-test. The results are reported as mean ± standard deviation (SD), and the differences observed between composites results were considered significant when p<0.05.
3. Results and Discussion
3.1. Characterization of Metabolites from Extract of Mimosa Tenuiflora
- M. tenuiflora has been used for wound healing and treatment of skin burns in Central and South America [12]. The high content of saponin and tannins is considered the cause of its healing properties due to antimicrobial, anti-inflammatory and cicatrizing effects [12, 22]. The dried bark of M. tenuiflora is highly rich in steroidal saponins [23]. The procedure used in this research to get the extract of M. tenuiflora was the one proposed previously by other researchers [12]. They found that the extract was composed mainly by arabinose, galactose (arabinogalactans), glucose, mannose and galacturonic acid. We also detected the presence of alkaloids, saponins, flavonoids, quinones and arabinogalactans (Figure 1). For this research, it was very important to find evidence of flavonoids because these metabolites possess a therapeutic potential in some human disorders. They exhibit an antioxidant activity inactivating free radical or preventing decomposition of hydroperoxides into free radicals. In addition, flavonoids exhibit antioxidative, antiviral, antimicrobial, antiplatelet and antitoxic activities [24]. Moreover, bioflavonoids have been associated with bone health and osteoblast differentiation in vitro [25]. On the other hand, the biological roles of alkaloids include both anti-inflammatory and antibacterial effects [26, 27]. Furthermore, some chromone alkaloids have been associated with osteoclast differentiation inhibitory activity that could promote a better bone regeneration in patients with osteoporosis [28]. Finally, the extract of M. tenuiflora had evidence of arabinogalactans. It had been found that arabinogalactans are potent stimulators of dehydrogenase activity and proliferation of skin fibroblasts, with a strong improvement in viability and proliferation [12]. Although it has been reported that the cortex contains phenols, tannins, steroids and triterpenoids [12, 29], we did not find any evidence of these secondary metabolites from the ethanol fraction obtained in our research.
3.2. Morphology and in Vitro Bioactivity of the Scaffolds
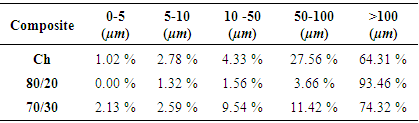
- Chitosan is a material with favorable degradability, antimicrobial activity, thermal stability and biocompatibility [30]. Nevertheless, the use of chitosan in tissue engineering has some limitations, including loss of bioactivity [31, 32]. The bioactivity could be measured when the material shows a kinetic modification in a surface layer during a certain period after the implantation [33]. In essence, it refers to inducing precipitation and mineralization of calcium phosphate on the surface [30]. This modification is a carbonated hydroxyapatite layer, which is chemically and structurally equivalent to a mineral phase in bone and provides an interface link between materials and tissues. In this way, the bioactivity of artificial materials could be attributed to the formation of an active layer of hydroxyapatite [33]. The process of formation of hydroxyapatite in bioactive materials can be reproduced in an SBF, which can predict the in vivo bone bioactivity. SBF has been used in different concentrations including from 1x until 5x [32, 34]. Its main advantage is that SBF is a biomimetic method that does not require a special equipment or process of high temperatures to produce a hydroxyapatite layer. The properties of this layer can affect the cell viability and proliferation [32]. The process of apatite formation requires consumption of calcium and phosphate ions from the surrounding body fluid. The calcium phosphate uses OHˉ, CO32ˉ, Na+, K+ and Mg2+ ions from the SBF solution until it crystallizes into hydroxyapatite [35].Scaffolds are used for bone tissue regeneration in order to provide an environment for the bone formation, including porous structures that are essential for cell migration, bone tissue ingrowth, vascularization and exchange of nutrients and waste [36, 37]. During this study, the composites obtained consisted of pores mainly in deep planes of the scaffold and few pores at the surface. Pores with sizes around 100 μm play an important role in bone ingrowth [8]. It has been estimated that optimal pore size varies from 150 to 500 µm [38]. The 80/20 composite (Figure 2) showed porosity around 100 µm and 500 µm and small pores between 20 and 80 µm (Table 1).
 | Figure 2. Micrographs of a) 80/20 chitosan/extract of M. tenuiflora and 80/20 chitosan/extract of M. tenuiflora incubated by (b) 21 days, (c) (d) and (e) 28 days at 100X and (f) 28 days at 5500X |
|
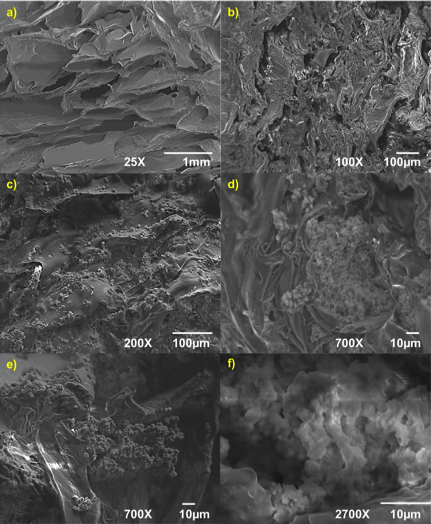 | Figure 3. Micrographs of a) 80/20 chitosan/extract of M. tenuiflora, and 80/20 chitosan/extract of M. tenuiflora incubated by (b) 21 days, (c) (d) and (e) 28 days at 100X and (f) 28 days at 5500X |
3.3. FTIR and XRD of the Samples
- Before the SBF treatment, the composites were immersed in tetraethoxylane (TEOS) to functionalize with the Si-OH groups. These groups act as a nucleation site to accelerate the formation of apatite crystals during the incubation in SBF [37]. During this research, SBF was used to promote that silanol groups attract calcium ions and the deposition of anions in SBF such as phosphate and carbonate [39]. The formation of apatite layer can be reproduced with a cellular SBF with ion concentrations nearly equal to the human blood plasma [31, 40, 41]. Thus, the chemical structure of the incubated composites was analyzed using FTIR. The chemical interaction could be appreciated by analyzing the changes in the characteristic peaks of the substance or the appearance of new peaks [42]. Regarding the hydroxyapatite layers in the FTIR analysis and in order to get a better definition of the hydroxyapatite bands, the original spectra of each composite were subtracted from the one obtained for each SBF treated composite (Figures 4 and 5). The IR analysis for the 80/20 composite revealed the presence of the v3 stretching mode of P-O bonds around 1063 cm-1 and 607 cm-1, which is evident at 28 days in all composites. The band at 1409 cm-1 provides evidence of carbonate (CO32) incorporated in the apatite [35]. On the other hand, 70/30 composite revealed bands at 1076 cm-1 corresponding to P-O bonds and bands at 847 cm-1 and 1417 cm-1 for CO3-2 bonds. The bands were more evident at 28 days. Therefore, it is possible to suggest that carbonated apatite was formed.
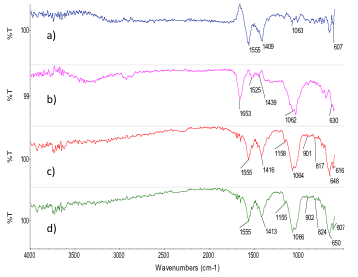 | Figure 4. FTIR of 80/20 chitosan/extract of from M. tenuiflora incubated in 1.5X SBF by (a) 7 days, (b) 14 days),(c) 21 days and (d) 28 days |
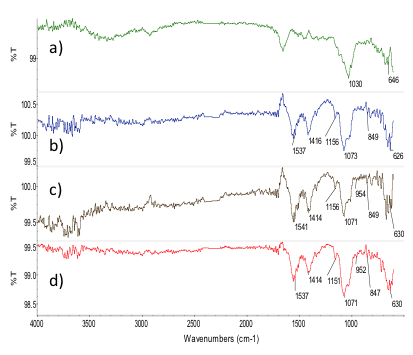 | Figure 5. FTIR of 70/30 chitosan/extract of M. tenuiflora incubated in 1.5X SBF by (a) 7 days, (b) 14 days), (c) 21 days and (d) 28 days |
- XRD patterns (Figure 6) indicate that the strength and width of HA gradually increased with the immersion time, increasing from 1 to 28 days. The XRD diffractograms of composites show a peak of high intensity at
 (Figure 8). This peak corresponds to the regular crystal lattice (1 1 0) of chitosan [43]. On the other hand, X-ray diffraction analysis confirmed the peaks of the chitosan/M. tenuiflora composite after incubation were sharper and stronger, which suggested that higher levels of crystallinity were formed on the scaffold apatite. The time for formation of apatite crystals on the surface is shown in the X-ray diffraction spectra on Figure 5. For the composites, a peak appearing at
(Figure 8). This peak corresponds to the regular crystal lattice (1 1 0) of chitosan [43]. On the other hand, X-ray diffraction analysis confirmed the peaks of the chitosan/M. tenuiflora composite after incubation were sharper and stronger, which suggested that higher levels of crystallinity were formed on the scaffold apatite. The time for formation of apatite crystals on the surface is shown in the X-ray diffraction spectra on Figure 5. For the composites, a peak appearing at  after immersion became less broad with respect to longer immersion times, suggesting the formation of apatite with higher crystallinity, as reported previously [19]. In addition, it is possible to identify peaks for amorphous calcium at
after immersion became less broad with respect to longer immersion times, suggesting the formation of apatite with higher crystallinity, as reported previously [19]. In addition, it is possible to identify peaks for amorphous calcium at  and
and 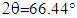 for CACO3. It is possible to identify a peak at 56° that corresponds to octacalcium phosphate [44]. Once apatite nuclei are formed, they can grow by consuming calcium and phosphate ions from the SBF solution. The Si-O- on the composite attracts a positive Ca layer, which then combines with negatively charged PO43- [37]. The relative intensities of apatite deposited on the surface of scaffold increased with an immersion time of the composite. Combined with the results of SEM/EDS and FTIR, it can be deduced that the induced apatite was a carbonated HA.
for CACO3. It is possible to identify a peak at 56° that corresponds to octacalcium phosphate [44]. Once apatite nuclei are formed, they can grow by consuming calcium and phosphate ions from the SBF solution. The Si-O- on the composite attracts a positive Ca layer, which then combines with negatively charged PO43- [37]. The relative intensities of apatite deposited on the surface of scaffold increased with an immersion time of the composite. Combined with the results of SEM/EDS and FTIR, it can be deduced that the induced apatite was a carbonated HA.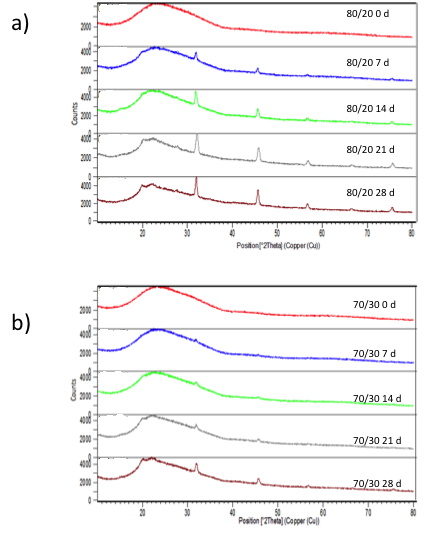 | Figure 6. XRD diffractograms of a) 80/20 and b) 70/30 chitosan/extract of Mimosa Tenuiflora composites before incubation and at 7, 14, 21 and 28 days after incubation in SBF 1.5X at 37º C |
3.4. In Vitro Degradation
- The degradation rate of scaffold for bone tissue engineering is considered a key factor. The degradation rate must match the ingrowth rate of newly formed bone [45]. In vitro degradation of Chitosan/Extract of M. tenuiflora composites were assessed by incubating them under physiological conditions and comparing the weight difference before and after each test. As shown in Figure 7, the weight of composites had the tendency to slowly decrease with incubation time. Primary amines capture protons from water and alkalize the culture medium. The pH of the medium was increased because of groups and alkaline ions, which are produced during the degradation of chitosan [46] and M. tenuiflora. The amino groups or hydroxyl groups of chitosan can neutralize the effects of the carboxyl groups of M. tenuiflora [16].
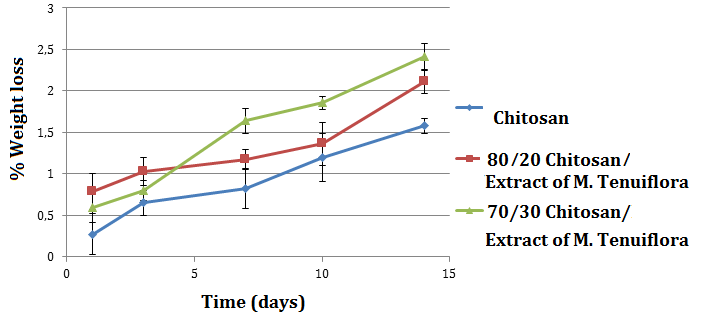 | Figure 7. Weight loss of composites of the composites |
3.5. Cell Viability and Proliferation
- The cell culture in vitro was used to assess the influence The cell culture in vitro was used to assess the influence of M. tenuiflora of the scaffolds on cell behavior. A bar graph for behavior of the cell number of osteoblasts seeded on different scaffolds is shown in Figure 8. At 72 h, the composite 80/20 Ch/Extract of M. tenuiflora shows a significant higher number of cells than the control and chitosan scaffold. After 72 h, the number of cells in 80/20 and 70/30 composites was similar within the same level of significance. These results suggest that the combination between Chitosan and M. tenuiflora allows increased viability in different combinations.
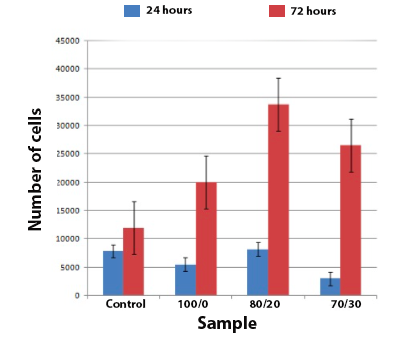 | Figure 8. Number of cells after culture 24 h and 72 h |
3.6. Biochemical and Differentiation Analyses
- During the stages in osteoblast differentiation, specific bone proteins are used to monitor osteoblastic phenotype [47]. The primary culture of osteoblasts can express specific proteins, such as ALP, osteocalcine and type I collagen, in the same temporal order like in vivo expression [48]. ALP activity is observed at early stages of osteoblast differentiation, particularly during the immature stage [47]. ALP has been associated with the mineralization process, so an increased level of the ALP is a sign of the metabolic activity of the osteoblasts [48].The bar graph in Figure 9a illustrates the results of the ALP activity of the cells in the scaffolds. All composites showed ALP expression, indicating that the osteoblasts were able to begin the differentiation process. The ALP expresión was significantly more in 80/20 composite than in other scaffolds. On the other hand, Figure 9b shows the DNA quantitation of the samples. DNA content results showed different cell proliferation behaviors on the different scaffolds. We noted no significant difference in the cell proliferation at 24 h among the scaffolds. More importantly, significant higher cell numbers were found on the 80/20 scaffolds at 72 h. Considering the number of the cells on all the scaffolds at 24 h were almost the same, the higher cell numbers on the 80/20 scaffolds indicated that better cell compatibility was achieved with this composition.
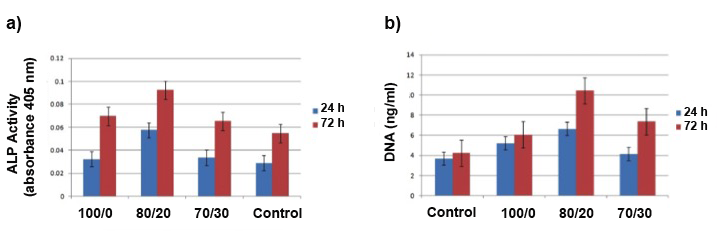 | Figure 9. a) ALP activity and DNA quantitation at 24 hours and 72 hours after cell culture |
- Figure 10 shows the presence of a large amount of globular mineral deposits attached to the scaffolds that was also confirmed by SEM micrographs and EDS analysis (Figure 4). This observation showed that a number of cells were well adhered to the scaffold.
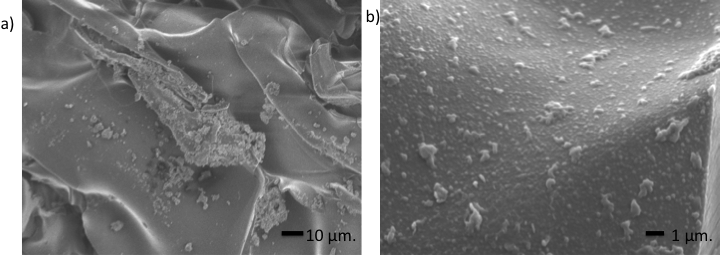 | Figure 10. Osteoblasts seeded of the 80/20 chitosan/extract of M. tenuiflora scaffolds |
4. Conclusions
- Chitosan/Extract of M. tenuiflora scaffolds were successfully prepared by thermally-induced phase separation technique. An accelerated process of biomineralization with concentrated SBF was used in the assessment of bioactivity of the composite. The chitosan/Extract of M. tenuiflora extract composites showed better biomineral activity than chitosan scaffolds. In the scaffolds, polysaccharides from M. tenuiflora provided nuclei in the mineralization process. As a result, more apatite was formed on the composite scaffolds than on the chitosan scaffolds. The ability of the composites to form a mineralized layer on their surface was evident by the cumulative results obtained from IR spectra, SEM and EDS. It was demonstrated that the Chitosan/extract of M. tenuiflora composites were able to induce the bone-like apatite nucleation and growth on their surfaces from SBF. In addition, it was demonstrated that the composites promote biocompatibility with osteoblasts cells.
ACKNOWLEDGEMENTS
- The authors acknowledge the financial support of the Mexican Public Education Secretary and the Mexican National Council for Science and Technology (CONACyT) through project SEP-CONACyT CB 2012-01-180909. Also, we greatly appreciate the support of B.S. Georgina Lopez during the management of the project.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML