-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2014; 4(5): 204-212
doi:10.5923/j.cmaterials.20140405.02
Development of Novel Wax-enabled Thermoplastic Starch Blends and Their Morphological, Thermal and Environmental Properties
Muhammad Pervaiz1, 2, Philip Oakley1, 3, Mohini Sain1, 4, 5
1Centre for Biocomposites and Biomaterials Processing, Faculty of Forestry, University of Toronto, Toronto, Canada
2Green Core Composites Inc., Sarnia, Canada
3The Delphi Group, Ottawa, Canada
4Centre of Advanced Chemistry, King Abdulaziz University, Jeddah, Saudi Arabia
5Mechanical Engineering Department, Luleå University of Technology, Luleå, Sweden
Correspondence to: Muhammad Pervaiz, Centre for Biocomposites and Biomaterials Processing, Faculty of Forestry, University of Toronto, Toronto, Canada.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Novel thermoplastic starch (TPS) melt-blends were prepared while using glycerol as plasticizer. To impart hydrophobicity, beeswax (BW) and paraffin wax (PW) polymers were introduced in combination with commonly available interfacial coupling agent, maleic anhydride (MA). Representative melt-blends of TPS containing different concentration of BW, PW and MA were prepared through in-situreactive extrusion in a custom designed twin screw extruder. The modified TPS was comprehensively evaluated for morphology, thermal stability and moisture resistance properties, while keeping both the temperature profile of extruder and glycerol concentration at constant level. The results showed that the plasticization becomes problematic at wax levels more than 10% at required extrusion temperatures due to lower melting points of these compounds, however FTIR studies exhibited an effective grafting of MAH compounds on wax polymers. Although phase separation was observed during morphological studies for BW-enabled TPS blends, but MA treated PW and their TPS blends showed homogenous structure of extruded samples. It was further observed that PW-enabled TPS blends had better thermal stability and enhanced hydrophobicity compared to BW-enabled formulations due to unique chemical structure of paraffin wax.
Keywords: Corn starch, Biopolymers, Thermoplastic starch, Biodegradable, Extrusion, Hydrophobic, Water absorption, Melt-blending of starch, Renewable resources
Cite this paper: Muhammad Pervaiz, Philip Oakley, Mohini Sain, Development of Novel Wax-enabled Thermoplastic Starch Blends and Their Morphological, Thermal and Environmental Properties, International Journal of Composite Materials, Vol. 4 No. 5, 2014, pp. 204-212. doi: 10.5923/j.cmaterials.20140405.02.
Article Outline
1. Introduction
- The development of biomaterials from renewable sources have gained considerable momentum in recent times due to unprecedented environmental and global climate change issues linked to mass-scale exploitation of petroleum-based feedstock [1-4]. Starch–based biopolymers from various plant sources present a viable alternative to develop biodegradable plastics for a variety of applications [5-7]. The main motivation to use starch as a suitable material for the production of biodegradable plastics is due to its low cost and abundant availability [8, 9]. Starch, produced naturally during photosynthesis in plantslike potatoes, corn, and rice, functions as the principal polysaccharide reserve material deposited in the form of granules [10-12]. The transformation of starch into bioplastic for any application needs disruption of granules through heating in the presence of plasticizers, usually glycerol and water, under specific conditions; the biopolymer thus produced is known as thermoplastic starch (TPS) [13-16]. Plasticized starch or thermoplastic starch (TPS) is prepared under specific extrusion conditions and in the presence of plasticizers, such as glycerol and water [14-16]. Although being compostable bioplastic, the mass-scale application of untreated TPS has been hindered largely due to its moisture absorbing ability at ambient environment [5, 8]. Sain et al. have reported a novel biodegradable modified TPS manufactured from a native starch using a polysaccharide produced by the fungus species Ophiostomaulmi. This modified TPS exhibits low water absorbency and high tensile strength and may be used to manufacture films or molding products by casting, extrusion, injection, or compression techniques [17]. In other works, researchers have tried to enhance the water resistance of TPS by melt-blending starch with hydrophobic polymers, such as poly(e-caprolactone) [18], cellulose acetate [19], poly (butylene adipate-co-terephthalate) [20], polylactides [21] and processing amylose-free corn starch with poly butylene succinate (PBS) [22]. However, the commercial application of these hydrophobic polymers have been restricted due to their high cost and poor functionality compared to commodity plastics. For the first time, this current work has investigated the potential of two common and low cost waxes, paraffin wax and beeswax, to be melt-blended with TPS in order to improve its water resistance.Waxes are hydrophobic organic compounds often used for waterproofing purposes in applications such as wax paper and wood composites [23]. Beeswax is a natural, hydrophobic, and biodegradable wax produced in the beehive by honey bees. The chemical nature of beeswax is basically lipoid, with the major components being 14% hydrocarbons, 35% monoesters, 3% diesters, and 12% free acids [24]. Previous studies on melt-blending esters with TPS have found that the mixtures are immiscible; however, they form compatible blends as a result of the hydrogen bonding interaction between the ester carbonyl group and the OH groups on starch [25], thereby, beeswax may show similar behaviour in the absence of any compatibilizer.Paraffin wax, a petroleum derived polymer, is composed of a mixture of alkanes ranging from 20 to 40 carbon chain-length. In order to improve the compatibility between starch and paraffin wax, a compatibilizer such as MAH, in the presence of dicumyl peroxide (DCP) as initiator, must be used when they are melt-blended [26]. The maleation reaction of paraffin wax, Figure 1, has been studied in the literature [27] and is believed that maleated paraffin wax will react with starch in a similar fashion to maleated polyethylene [28].
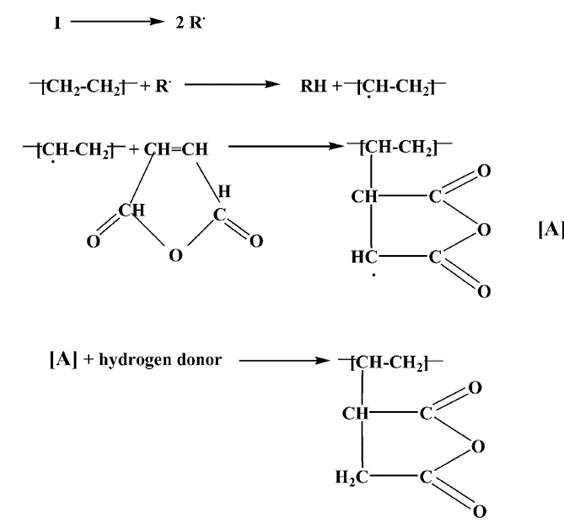 | Figure 1. Reaction scheme for grafting maleic anhydride onto paraffin wax [27] |
2. Experimental
2.1. Materials
- Industrial grade cornstarch (11% moisture) was obtained from Casco Inc. (Cardinal, ON., Canada). Glycerol was purchased from ACP Chemicals Inc. (Montreal, QC, Canada). Beeswax (BW), paraffin wax (PW, Tm = 80-90oC), maleic anhydride (MAH), and dicumyl peroxide (DCP) were purchased from Sigma-Aldrich (Oakville, ON, Canada).
2.2. Plasticization
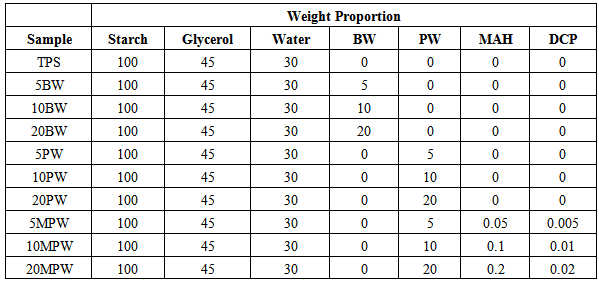
- Starch and glycerol were mixed with a high speed kitchen mixer for 30min. BW or PW or PW, MAH, and DCP were added and mixed for an additional 10min. The compositions of ten samples prepared are listed in Table 1.The plasticization was done through A twin screw extruder, ONYX TEC 25/40, available at Center for Biocomposites and Biomaterials Processing (CBBP), University of Toronto, Canada, was used for plasticization with a temperature profile along the extruder barrel (from feed zone to die) as given in Table 2.
|
|
2.3. FTIR
- The functional groups of PW and MPW were identified through use of a Bruker Tensor-27 spectrometer. All spectra were captured over a range of 400 to 4000 cm-1 at a resolution of 4 cm-1 with 200 scans. To determine the grafting of maleic anhydride, paraffin wax was extruded separately at the same processing conditions and in the presence of MAH and DCP. The extrudate was purified to remove any unreacted MA by dissolution of PW in boiling water for 10min, followed by vacuum filtration.
2.4. SEM
- The exposed surfaces of specimens, fractured through a sharp-edge knife, were coated with gold and observed with a JEOL JSM-840 scanning electron microscope (Tokyo, Japan). The electron gun voltage was set at 15 kV. The micrographs were arranged at 200x magnifications to observe surface morphology.
2.5. TGA
- The thermal behaviour of TPS blends was studied with a TGA Q500 type thermal analyzer (TA Instruments - New Castle, DE, USA). Samples, weighting from 1 – 5 mg, were heated on a platinum pan from ambient temperature to 600°C at a rate of 15°C/min. Derivatives of TGA were obtained using TA Instruments Universal Analysis software.
2.6. Water Absorption
- Water absorption (WA) of TPS blend-extrudates was determined by preparing 2x2square inch thin film specimens using hydraulic press, ARG-450-Dieffenbacher N.A. Inc. Windsor - Canada, followed by hot pressing for 4min at 160℃ and 500kPa, cut into square specimens, and dried overnight in a desiccator. Dried specimens were placed in a desiccator containing distilled water at room temperature (23℃, 100% RH) and weighed every 24h. WA of each specimen was calculated by the following equation:
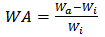 | (1) |
3. Results and Discussion
3.1. FTIR
- The different methods of grafting of maleic anhydride and its isostructural analogues on natural and synthetic polymers have been discussed in detail in literature [26,29]. Reactive extruder systems and in-situ compatibilazation for grafting of MAH onto various thermoplastic polymers, especially polyolefins, to prepare high performance engineering materials have been significantly developed [30]. For the maleated wax, the characteristic FTIR absorption band has been reported at 1790 cm-1, corresponding to C=O of five-membered cyclic anhydride. [31]. The unreacted MAH, if any, is usually represented by the peak at 698cm-1, attributed to the C=C bond in MAH [32]. Figure 2 shows the spectra for unreacted paraffin wax and maleated paraffin wax. The spectrum for the unreacted wax, as anticipated, shows no peaks at 1790cm-1 and 698cm-1, eliminating the chance of any succinic anhydride or maleic anhydride presence in the sample. On the other hand, a prominent characteristic peak at 1790cm-1 is visible in the spectrum of maleatedwax (MPW), confirming the grafting of MAH onto the wax in the form of succinic anhydride. Further, absence of any peak at 698 cm-1 in same spectrum rules out possibility of any unreacted MAH in maleated wax sample.
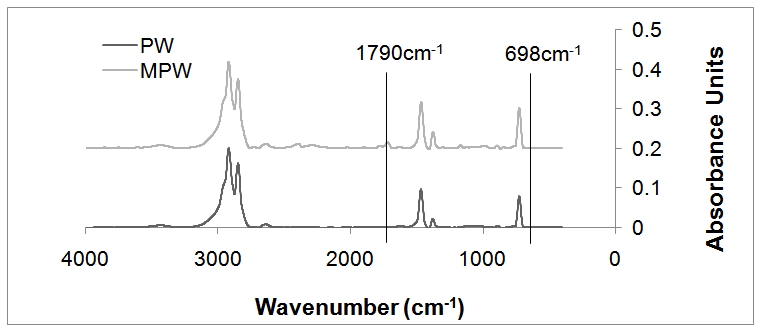 | Figure 2. FTIR spectrum for PW and MPW |
3.2. Morphology Study
- Previous works have reported that the morphology structure of TPS depends to a great extent on the starchtype and plasticizer amount used [33, 34]. Micrographs of the fragile fracture surface obtained by SEM of BW-enabled TPS samples are shown in Figure 3. Some unplasticized starch particles were removed from the surface of the blends during the fracture of the specimen, leaving some pits in the fractured surface, as visible in Figure 3a. The mechanism of anti-plasticization effect of plasticizer on TPS has not been fully understood, but some efforts have been made to explain its mechanism [35]. Zhang & Han (2010) [36] observed that starch retrogradation results in antiplasticization phenomenon. At temperatures above glass transition, retrogradation process in TPS is pronounced with the evolution of the crystallinity and rearrangements of plasticizer molecules into the material, thus, reaction kinetics depending largely on the macromolecules mobility, on the plasticizer type and content [37]. It seems BW may have interfered with starch plasticization as well and there also appears a phase separation between TPS and BW, especially at higher BW content as shown in Figure 3b. Unfortunately, to the best of authors’ knowledge, no effort has been made so far to develop beeswax-enabled melt-blends of TPS, although use of beeswax as an auxiliary water-repelling film on TPS has been reported with encouraging results [38].
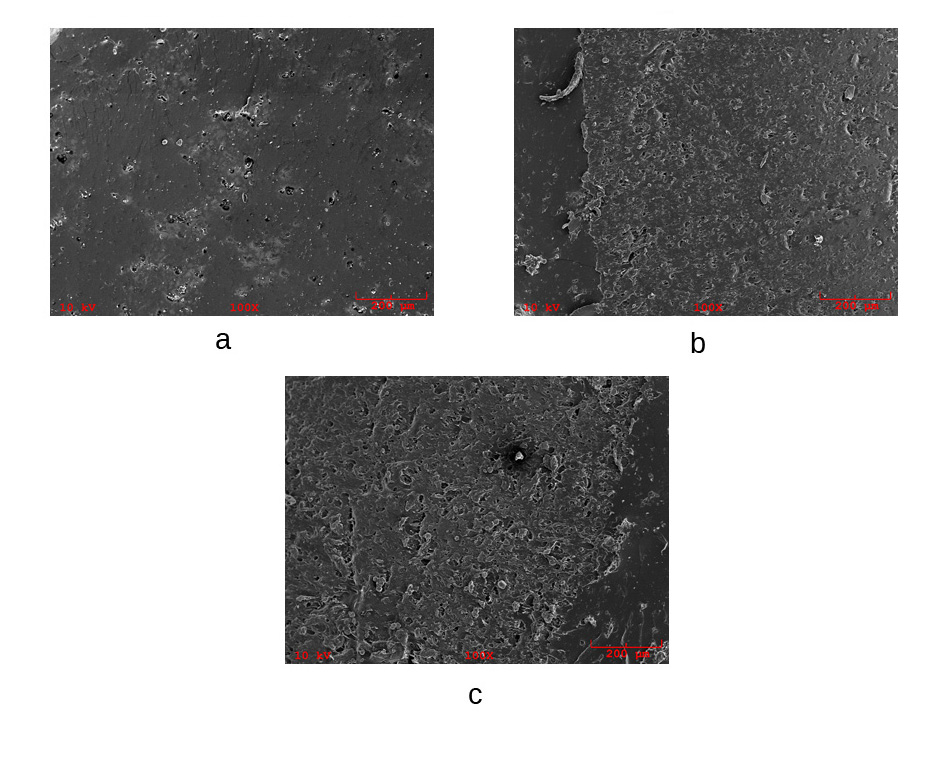 | Figure 3. SEM images of extruded samples: (a) 5BW (b) 10BW |
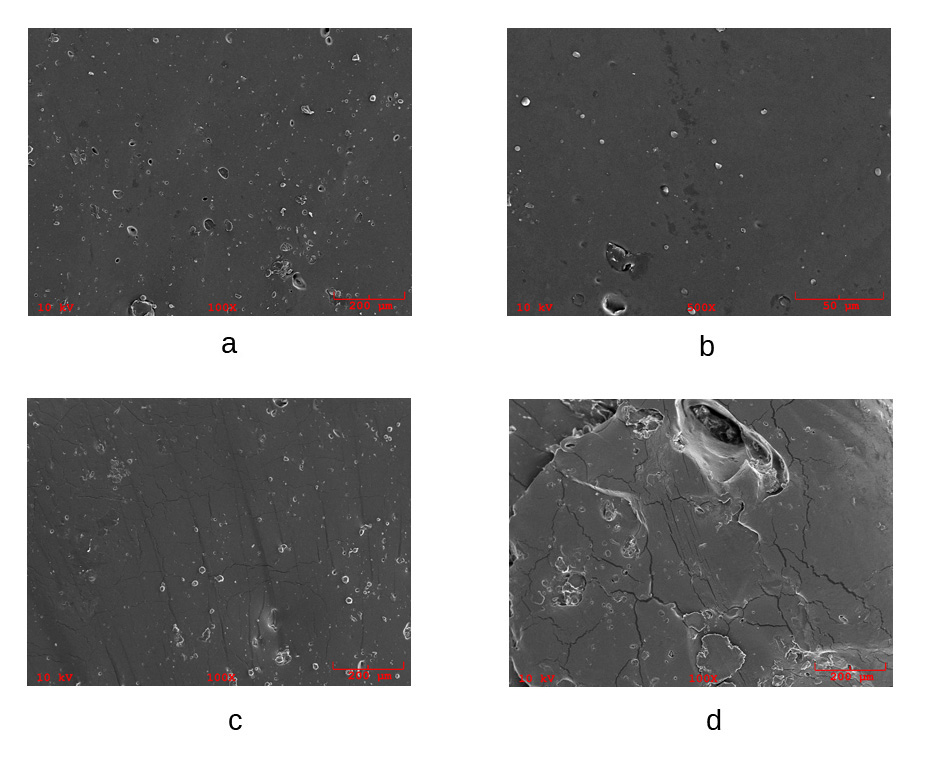 | Figure 4. SEM images of extruded samples: (a) 5PW (b) 10PW (c) 5MPW (d) 10MPW |
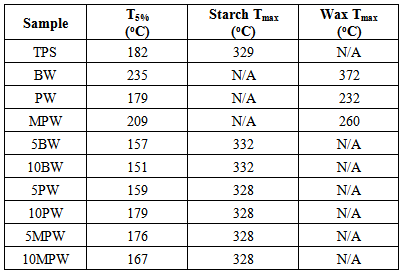
3.3. Thermal Analyses
- TGA curves for all wax/TPS samples are shown below in Figure 5 (a,b,c) with wax and TPS as references. In the case of all BW, PW, and MPW samples, there were two well defined shifts in the TGA curve. First, at around 100℃, water evaporation caused the initial weight loss. Weight loss continued gradually as water continued to evaporate along with glycerol (starting at 150℃), and then wax (at 150-200℃). A second major shift occurred from 300oC to 350℃ where the thermal degradation of starch occurred.
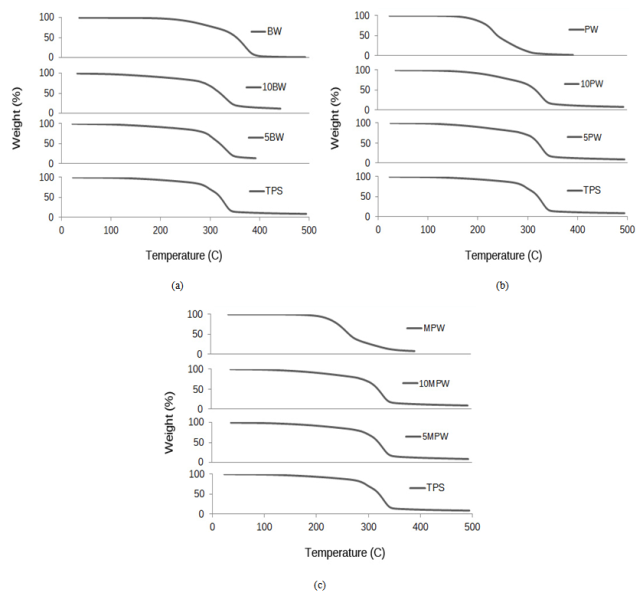 | Figure 5. TGA curves for pure BW, TPS and blends of TPS/BW (a), pure PW, TPS and blends of TPS/PW (b), MPW, TPS and blends of TPS/MPW (c) |
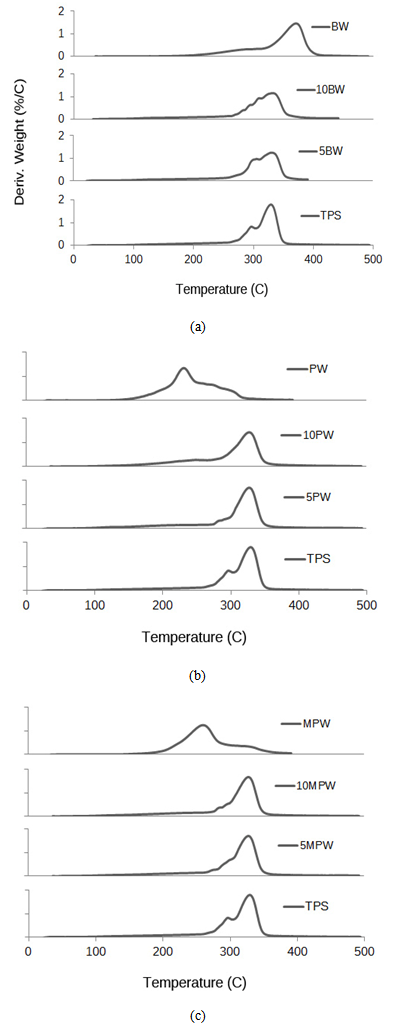 | Figure 6. Derivative TGA curves for pure BW, TPS and blends of TPS/BW (a), pure PW, TPS and blends of TPS/PW (b), MPW, TPS and blends of TPS/MPW (c) |
|
3.4. Water Absorption
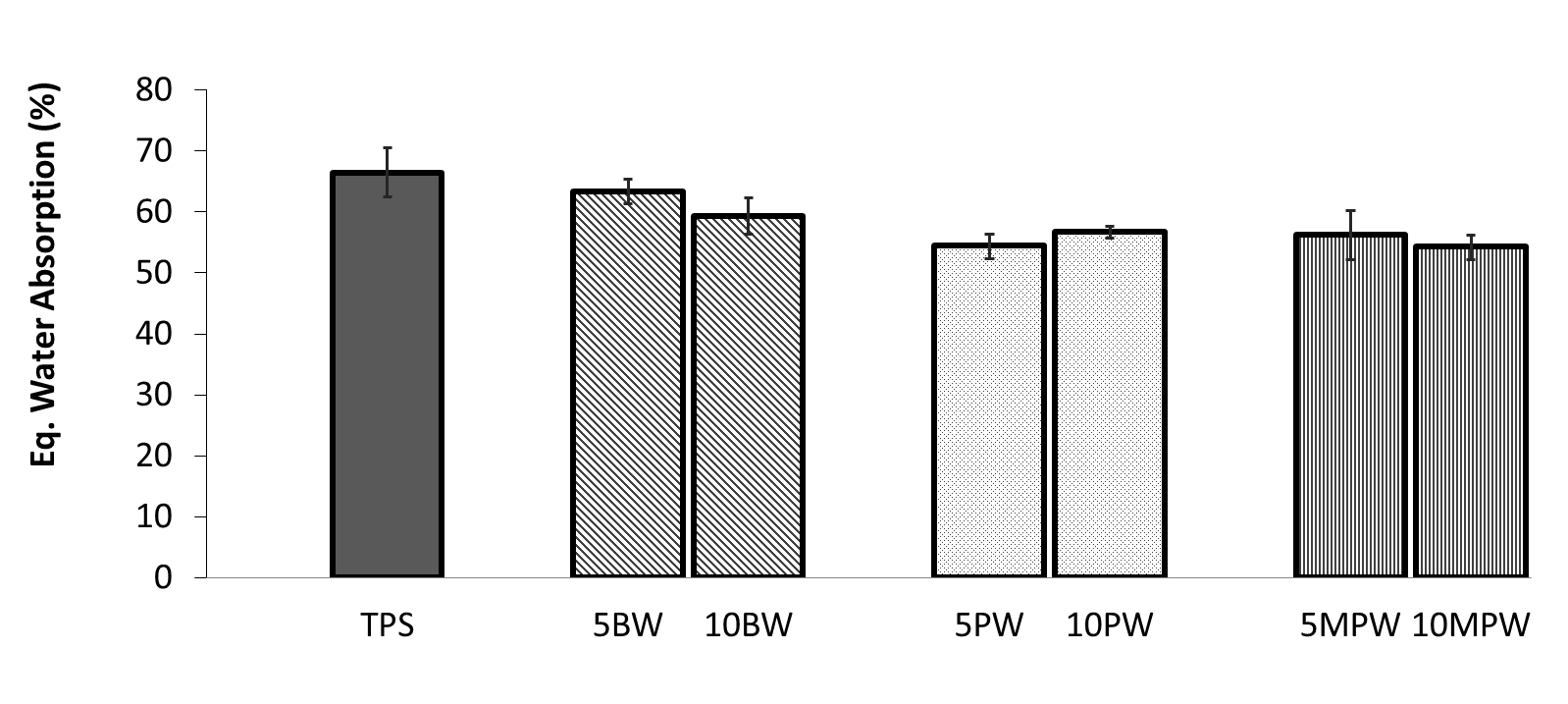
- Figure 7 shows the water absorption results for all TPS/wax blended samples with TPS as a reference. The tested value for TPS water absorption (66%) is comparable to the literature value (62%) for glycerol plasticized (50wt%) TPS [40].
 | Figure 7. Water absorption at equilibrium for TPS and TPS/Wax formulations |
3.5. Conclusions
- TPS formulations with beeswax and paraffin wax were developed through melt blending and reactive extrusion facilitated by MAH and DCP. The effect of different weight fractions of BW and PW were studied for physico-chemical properties while keeping glycerol level at constant levels. Also studied was effect of maleated PW over a range of concentrations and, subsequently, comprehensive morphological, thermal and hygroscopic analyses demonstrated some predicted and also novel results. l Morphological studies through SEM micrographs consistently showed some unplasticized areasowing to single-feeder system and retrogradation phenomenon, a common drawback involving rearrangements of plasticizer molecules into the starch material.l Phase separation was observed in BW-enabled TPS extrudated samples, whereas, PW-enabled TPS blended systems’ morphology showed much better and homogenous interfacial phase even in the absence of compatibilizer, MAH. The main reason for this could be attributed to different chemical structures of both waxes; BW being rich in esters while PW containing mostly homologous series of hydrocarbon chains. l The grafting of MAH as succinic anhydride on PW was confirmed through FTIR spectra as supported by literature examples; although MPW-enabled TPS blends did not exhibit any better surface morphology compared to PW-enabled blends, but their thermal performance improved compared to other formulations. l Thermal analysis during derivative TGA curves validated the enhanced stability of PW and MPW blends of TPS compared to BW blends; a phenomenon substantiated through SEMs as well by showing no phase separation of said formulations.l Although some reduction in moisture absorption was observed for TPS/BW blends during hygroscopic studies, but it was not significant compared to neat TPS; main reasons being loss of some uncontrolled amount of BW during sample preparation and presence of water-attracting esters in its chemical structure.l A significant reduction of 20% in moisture uptake was observed for PW blended TPS extrudates due to favorable chemical structure of paraffin wax. The differences in surface chemistry and physical structure of the waxes appear to be critical for the improvement of hydrophobicity of TPS blends. l The results of this study could be served as benchmark in developing moisture resistant bioplastics from renewable resources.
ACKNOWLEDGEMENTS
- This research project was made possible through financial support of Natural Sciences and Engineering Research Council of Canada-Collaborative Research and Development Grants (NSERC-CRD) and Ontario Research Fund: Research Excellence (ORF-RE) programs.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML