-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2014; 4(5): 197-203
doi:10.5923/j.cmaterials.20140405.01
An Experimental Study of the Effect of Nickel with SiC Codeposited on Aluminium 7075 under Direct Current and Pulse Current
Pradeep Devaneyan S.1, T. Senthilvelan2
1Research Scholar, Department of Mechanical Engineering, Pondicherry Engineering College, Puducherry, 605 014, India
2Professor, Department of Mechanical Engineering, Pondicherry Engineering College, Puducherry, 605 014, India
Correspondence to: Pradeep Devaneyan S., Research Scholar, Department of Mechanical Engineering, Pondicherry Engineering College, Puducherry, 605 014, India.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
In the present work, Nickel with micro SiC composite coatings were obtained by electrochemical codeposition method from an additive – free Watts type bath. The aim of this work is to obtain Nickel with micro SiC composite coatings on Aluminium 7075 with more reinforcements. For comparison, pure Nickel deposits were also produced under the same experimental conditions. The effects of the SiC particle concentration on the bath, addition of surfactants and the coating time on the composition of micro-composite coatings were studied. The surface morphology and micro structural study were executed by means of Scanning Electron Microscope (SEM) and X-ray diffraction (XRD) analysis. Characterization experiments showed micro SiC codeposition with Nickel caused changes in the texture of the Nickel matrix. Moreover, the results clearly depict the high incorporation of micro SiC in Pulse Current codeposition when compared with conventional Direct Current.
Keywords: Electro-codeposition, micro SiC, Direct Current, Pulse Current, SEM, XRD, Aluminium 7075
Cite this paper: Pradeep Devaneyan S., T. Senthilvelan, An Experimental Study of the Effect of Nickel with SiC Codeposited on Aluminium 7075 under Direct Current and Pulse Current, International Journal of Composite Materials, Vol. 4 No. 5, 2014, pp. 197-203. doi: 10.5923/j.cmaterials.20140405.01.
Article Outline
1. Introduction
- Applications of Aluminium 7075 gained increasing attention in aerospace and allied fields due to its inherent lightness and good strength-to-weight ratio. It replaces steel and many other metals where weight is the major factor since its density is one third of the steel. Even though it has good strength-to-weight ratio, it cannot withstand fretting wear and fretting fatigue loads as much as other metals [1-5]. Hence, when Aluminium is used under fairly high temperatures and weary conditions such as engine cylinder pistons, it needs surface treatments to increase the wear resistance and to lower the coefficient of friction. This is the fact which increased the interest on electrodeposition of Nickel on Aluminium 7075 that can minimize the problems of wear to some extent [3-7]. Therefore the process of electroplating has been carried out on applications such as engine cylinders, high pressure valves, drill fittings, aerospace, mining equipment where high wear rate can be spotted [16-20]. Electroplating of Nickel on any metal reduces wear but, when fine particles of micro size metallic or non-metallic reinforcement in Nickel plating enhances the wear resistance, lubrication and corrosion resistance than the pure Nickel plating [6-10].In the current scenario, research on codepositing ceramic particles such as SiC, Cr2O3, TiO2, Al2O3, and WC along with Nickel plating finds a platform to improve the wear resistance of plated component [6-20]. Improvement of wear resistance by this codeposition could be obtained only by optimizing the parameters such as percentage of particle concentration, bath composition and temperature and further more. Electro deposition of Nickel was conventionally adapted for many decades and was successfully implemented in the industries when compared with other techniques. Hence this research focuses on the optimization of the parameters to codeposit the non metallic particles along with Nickel using the conventional electroplating method.Recent literatures reveal that codeposition of micro size particles homogeneously in the deposit is highly difficult because of the higher tendency of micro particles to get agglomerated. Non-homogenous electro-codeposition leads to decrease the wear resistance of the coated substrate [21]. Hence more attention for research is needed to attain the homogeneous distribution of reinforced particles in near non- agglomerated form leads to the harder and more wear resistant coatings. Direct current (DC) was conventionally used to coat Nickel in industries but only few researches were carried out using Pulse Current (PC) to study the influence of it on electro-codepostion [22]. The aim of this research work is to find the suitability of electro-codeposition method by analysing the DC and PC parameters in order to attain the maximum incorporation and homogeneous distribution and thereby enhancing the wear resistance of the coatings.
2. Materials and Method
2.1. The Electrolyte
- The standard Watts’ Nickel Sulphate bath was used and the composition of plating solution along with the parameters was stated in Table 1. Codeposition of micro SiC in near non-agglomerated condition is the major problem in composite coatings. To avoid agglomeration problem Sodium Dodecyl Sulphate has been added as the surfactant which activates the positive zeta potential of micro SiC particles. The positive zeta potential of inert SiC particles gains extra adhesion force with the cathode is activated [23]. During electro-codeposition, the SiC particles settle at the bottom of the plating bath which reduces the percentage of incorporation of micro SiC particles in the codeposition process [25]. Along with the surfactant and stirrer, the agglomeration of the particles were avoided by preparing a mixture by blending the required volume of SiC with little electrolyte and a small volume of surfactant as a paste. The paste is then mixed in the plating bath and stirred for a minimum of one hour before deposition process.
|
2.2. Substrate Preparation
- The aluminium 7075 substrate does not have the tendency to get coated with Nickel deposition directly. Hence the Aluminium 7075 substrate has to undergo some preliminary process such as zincating and copper plating, before carrying out electro-deposition of Nickel with SiC. Aluminium 7075 was purchased in form of rods and was machined in the form of billets of size 25 mm diameter and 25 mm height as per the ASTM standards [6]. They were polished with sandpaper, 2000 grit size to obtain uniform and smooth surface. The aluminium substrates were first degreased before electroplating in 42 g/L NaOH solution at 65℃ for 20 seconds. Further the substrate is cleaned in distilled water and it is zincated (Zinc oxide 100g/L, Sodium hydroxide 525 g/L, ferric chloride 10g/L and Sodium tartate 1 g/L). Copper deposition is done on the zincated Aluminium substrate for 30 seconds and again washed in distilled water before the codeposition of Nickel with micro SiC is done [24].
2.3. Plating and Testing Methodology
- Nickel plate of 30 X 35 mm is used as anode. It was cleaned properly to remove the oxide layers. The bath which is shown in Fig. 1 was controlled by a tailor made microcontroller and it was developed for this research work through which all the parameters such as current density, voltage, plating time, temperature and stirring speed were controlled. The microcontroller was also equipped to generate DC and the PC with varying duty cycles. The microcontroller was designed in such a way that the parameters can be edited. The actual values and the edited values can be monitored through the display devices which were controlled by the microcontroller. The freshly prepared electrolyte as per the Table 1 is used and for fabrication of codeposited samples the SiC is dispersed in the form of paste and mixed to maintain the homogeneity using stirrer for minimum of one hour before the electro-codeposition. Pure Nickel and composite Nickel with SiC coatings were electrodeposited based on the conditions shown in Table 2. The commercial micro SiC powder of 99.9% pure with mean diameter of 2-3µm was added in the bath. The pH of the plating bath was maintained to 4.0 ±0.1 in all conditions. A constant distance of 3 cm was maintained between the cathode and the anode. The current density, voltage and temperature were constantly maintained and the values have been furnished in table 2 for all samples. Both the DC and PC with 50% duty cycle were employed to coat the samples and it was ultrasonically cleaned in ethanol for 10 minutes to remove loosely adsorbed particles from the surface. The coating time for each specimen was varied to maintain the coating thickness to a constant value of 50 µm. The composition of percentage weight of micro SiC was varied between 0% to 20% for both DC and PC. The samples were then prepared for SEM analysis. Morphology and crystallographic structures of the electro-codeposited samples were taken using SEM (JEOL-JSM-6610LV) machine at different magnifications. The coated samples were examined and the presence of micro SiC as reinforcement in the Nickel matrix was confirmed. The size of micro SiC incorporated in each codeposited samples were measured and it is correlated with the size of micro SiC dispersed in the plating solution.
|
 | Figure 1. The Experimental Setup |
3. Results and Discussion
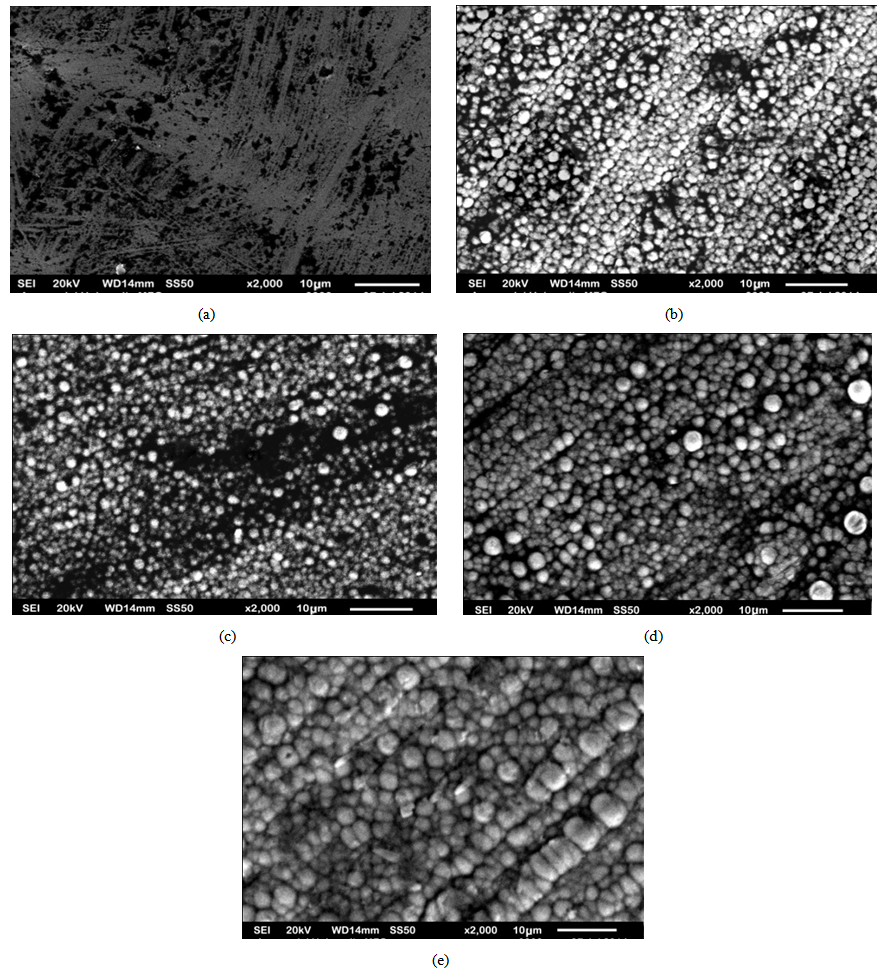
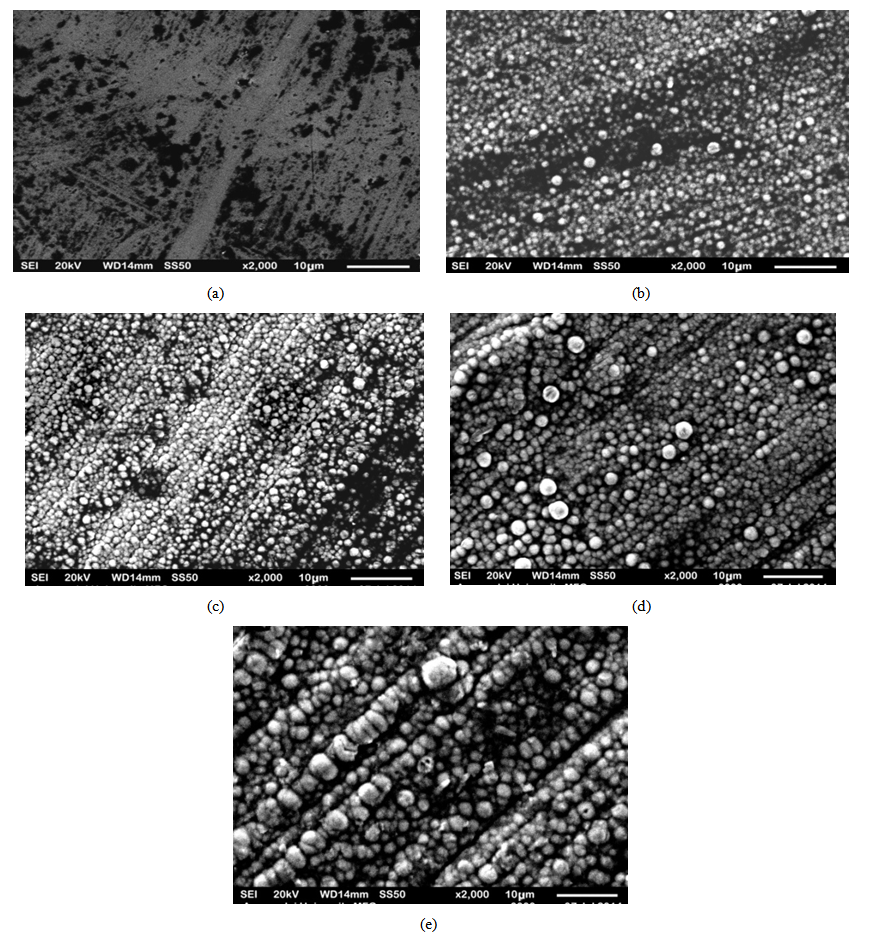
- The optimum values of the variables of the bath such as current density, voltage, coating time, percentage volume of the surfactant, stirring speed and the distance between cathode and anode were determined after conducting several experiments on trial basis. The smooth codeposited coatings as well as the codeposition of SiC were achieved under the condition of 4.0 pH, 55℃ temperature, 2 A/dm2 current density, 0.2 g/L surfactant and 2 V voltages. The surface morphology of the samples prepared by DC and PC were shown in Fig. 2(a-e) and Fig. 3(a-e). Fig. 4 (a and b) shows the XRD analysis result which was carried out with a Rigaku D/Max/2200/PC model X-ray diffractometer at a scanning speed of 1°/min in the 2 theta range of 20-80. The Fig. 2 (a) shows the pure Nickel coating and Fig. 2 (b), (c), (d), (e) shows the codeposition of SiC with Nickel in 5%, 10%, 15%, 20% under the application of DC. Fig. 3 (a) shows the pure Nickel coating, Fig. 3 (b), (c), (d), (e) shows the codeposition of micro SiC with Nickel in 5%, 10%, 15% and 20% under the application of PC. It is apparent from the Fig. 2 (b), (c), (d) and Fig. 3 (b), (c), (d) that the composite coatings which were obtained by 5-10% of SiC by both Direct and PC method is smooth with uniformly distributed micro SiC particles. Whereas, the agglomeration and irregular microstructure of the Nickel and micro SiC particles can be seen in Fig. 2(e) and Fig. 3(e).
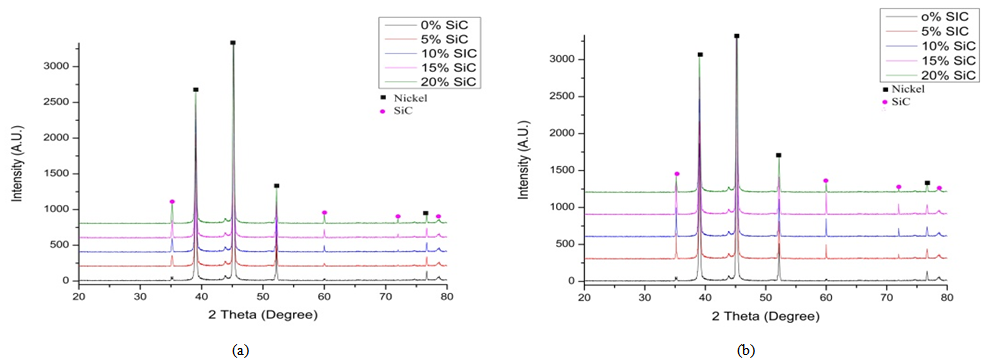 | Figure 4. XRD patterns of (a) Direct Current (b) Pulse Current |
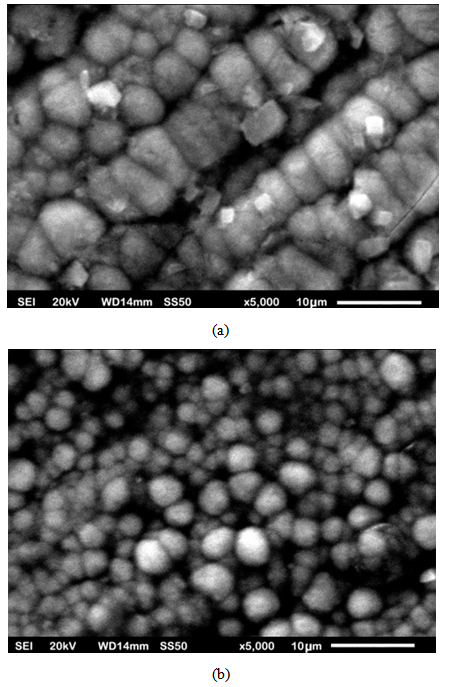 | Figure 5. SEM micrographs of the (a). Agglomerated SiC particles (b). Uniformly codeposited SiC particles under same operating parameters |
4. Conclusions
- In this work, Nickels reinforced with micro sized (2-3µm) SiC particles were successfully codeposited by DC and PC electroplating. The influence of the concentration of SiC particles in the bath on its codeposition mechanism along with Nickel was investigated. The following observations have been made.1. The SEM micrographs were captured and it inferred that the PC electroplating is the best route to codeposit micro SiC with much higher weight percentage when compared with the DC electroplating. 2. The increase in concentration of SiC in the bath increases the codeposition in Nickel matrix and it reduces beyond the optimum level. 3. The addition of surfactant increases the codeposition and maintains the homogeneity of coating by improving the zeta potential. 4. Optimization of the parameters such as current density, temperature, pH value, % volume of SiC dispersed in the bath also leads to the uniform coating and maximizes the codeposition.
ACKNOWLEDGEMENTS
- The authors gratefully acknowledge the Annamalai University, Chidambaram for assisting to carry out the Scanning Electron Microscopy work. The authors would like to express their thanks to Ikon Associates, PIPDIC Industrial Estate, Puducherry for their support in carrying out the experimental work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML