| [1] | Charlie Sia Chin Voon, “Development and fabrication on the oil palm fiber/glass reinforced in aluminium composite laminates” M.Engr. thesis, University of Malaysia Sarawak, 2008. |
| [2] | (a) Matthew Lee Clingerman “Development and Modelling of electrically conductive composite material” Ph.D. thesis, Michigan Technological University, 2001. (b) ZHENG Jing, SONG Wen-juan, MA Guang, JIA Zhi-hua, “Thermal expansion and mechanical properties of Al/Si composites fabricated by pressure infiltration”, Trans. Nonferrous Met. Soc. China, vol. 17,p 326-329, 2007. (c) Gupta, V.K., et al., Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes, Water Research, 2011. (d) A Lj Bojic, M Purenovic and D Bojic “Removal of chromium (VI) from water by micro-alloyed aluminium composite (MAlC) under flow conditions” Water SA Vol. 30, No. 3, p 353-359, 2004. (e) Hossein Eisazadeh “Removal of Arsenic in Water Using Polypyrrole and its Composites” World Applied Sciences Journal , vol. 3 (1), p 10-13, 2008. (f) Mahoko Murakami, “Surface properties of an indirect composite polymerized with five laboratory light polymerization systems,” Journal of Oral Science, Vol. 51, No. 2, p215-221, 2009. (g) Qingfeng Tan, Xiaoun Bao, Tengchun Song, Yu Fan, Gang Shi, Baojian Shen, Conghua Liu and Xionghou Gao, “Synthesis, characterization, and catalytic properties of hydrothermally stable macro–meso–micro-porous composite materials synthesized via in situ assembly of preformed zeolite Y nanoclusters on kaolin” Journal of catalysis, vol.251,1, p 69–79, 2007. (h) Shuilin Zheng et al., “Preparation and Photocatalytic Property of TiO2/Diatomite-Based Porous Ceramics Composite Materials” International Journal of Photoenergy, Article ID 264186, 2012. (i) L.C.Davis,J. Chen and M.F.Thorpe, “Predicting the elastic properties of Composite materials”, Proceedings of the American society for composites-seventh technical conference, oct.13-15, 1992. |
| [3] | C. Cannas, D. Gatteschi, A. Musinu, G. Piccaluga, C. Sangregorio, "Structural and Magnetic properties of Fe2O3 Nanoparticles Dispersed over a Silica Matrix", J. Phys. Chem. B,vol. 102, p7721,1998. |
| [4] | C. Cannas, G. Concas, A. Musinu, G. Piccaluga, G. Spano, “Mossbauer spectroscopic study of Fe2O3 nanoparticles dispersed over a silica matrix”, Naturforch., vol.54a, p513, 1999. |
| [5] | D. D. Awschalom, D. P. DiVicenzo, , “Complex Dynamics of Mesoscopic Magnets", Phys. Today, vol. 48, 4,p 43 1995. |
| [6] | R. F. Ziolo, E. P.Giannelis, B. A. Weinstein, M. P. O’Horo, B. N. Ganguly, V. Mehrotra, M.W. Russell, D. R. Huffman, , “Matrix-Mediated Synthesis of Nanocrystalline -Fe2O3: A New Optically Transparent Magnetic Material", Science, vol. 257, p219, 1992. |
| [7] | R. D. McMichael, R. D. Shull, L. J. Schwartzendruber, L. H. Benett, R. E.Watson, “Magnetocaloric effect in superparamagnets” J.Magn.Magn.Mater., vol. 111,29, 1992. |
| [8] | T. Ida, H. Tsuki, A. Ueno, K. Tohoji, Y. Udagawa, K. Iwai, H. Sano, “characterization of iron oxide in Fe2O3 SiO2 catalyst” J. Catal., vol. 106, p428 ,1987. |
| [9] | C. Cantalini, M. Pelino, “Microstructure and humidity- sensitive characteristics of α-Fe2O3 ceramic sensor” J.Am. Ceram. Soc., vol.75, p546, 1992. |
| [10] | C. Cantalini, H. T. Sun, M. Faccio, G. Ferri, M. Pelino, “Niobium-doped a-Fe2O3 semiconductor ceramic sensors for the measurement of nitric oxide gases” Sensors and Actuators B, vol.24-25, p673 1995. |
| [11] | H. T. Sun, C. Cantalini, M. Faccio, M. Pelino, M. Catalano, L. Tapfer, “Porous silica-coated a-Fe2O3 ceramics for humidity measurement at elevated temperature” J. Am. Ceram. Soc., vol.79, p927, 1996. |
| [12] | S. Roy, D. Das, D.Chakravorty, D. C. Agrawal, “Magnetic- properties of glass-metal nanocomposites prepared by the sol-gel route and hot-pressing” J. Appl. Phys. Vol.74, p4746, 1993. |
| [13] | Le Zeng, “A method for preparing silica-containing iron (III) oxide adsorbents for arsenic removal” Water Research, vol.37, p 4351–4358, 2003. |
| [14] | T. Tago, T. Hatsuta, K. Miyajima, M. Kishida, S. Tashiro and K. Wakabayashi, “Novel Synthesis of Silica-Coated Ferrite Nanoparticles Prepared Using Water-in-Oil Mi-croemulsion,” Journal of the American Ceramic Society, Vol. 85, No. 9,p. 2188-2194, 2002. |
| [15] | A.-L. Morel, S. I. Nikitenko, K. Gionnet, A. Wattiaux, J. Lai-Kee-Him, C. Labrugere, B. Chevalier, G. Deleris, C. Petibois, A. Brisson and M. Simonoff, “Sonochemical Approach to the Synthesis of Fe3O4@SiO2 Core-Shell Nanoparticles with Tunable Properties,” ACSNANO, Vol. 5, No. 2, p. 847-856,2008. |
| [16] | Z. l. Lei, Y. L. Li and X. Y. Wei, “A Facile Two-Step Modifying Process for Preparation of Poly (SStNa)- Grafted Fe3O4/SiO2 Particles,” Journal of Solid State Chemistry, Vol. 181, No. 3,p. 480-486,2008. |
| [17] | Meizhen Gao, Wen Li, Jingwei Dong, Zhirong Zhang, Bingjun Yang “ Synthesis and Characterization of superparamagnetic Fe3O4@SiO2 Core-Shell Composite Nanoparticles” World Journal of Condensed Matter Physics, vol. 1, p49-54, 2011. |
| [18] | C. Z. Huang and B. Hu, “Silica-Coated Magnetic Nanoparticles Modified withGamma-Mercaptopropyltrimethoxysilane for Fast and Selective Solid Phase Extraction of Trace Amounts of Cd, Cu, Hg, and Pb in Environmental and Biological Samples Prior to Their Determination by Inductively Coupled Plasma Mass Spectrometry,” Spectrochim Acta Part B: Atomic Spectroscopy, Vol. 63, p. 437-444, 2008. |
| [19] | Surender Duhan & Sunita Devi “Synthesis and Structural characterization of Iron Oxide-silica Nanocomposites Prepared by the Solgel Method” International Journal of Electronics Engineering, vol.2(1), p. 89-92,2010. |
| [20] | Zulfikar, M.A., A.W. Mohammad and N. Hilal. Preparation and characterization of novel porous PMMA-SiO2 hybrid membranes. Desalination vol.192,p262-270,2006. |
| [21] | S. Duhan, P. Aghamkar, M. Singh, N. Kishore, P. K. Sen, J. Sol-Gel Sci Tech., vol.46, 17,2008. |
| [22] | Mohammed Jasim uddin, Muhammed Shah Miran and Mohammad Yousuf Ali Mollah “Electrochemical Synthesis and characterization of iron oxyhydroxide” Journal of the Bangladesh Chemical Society, vol. 20(1),p39-45,2007. |
| [23] | Piccaluga G., Corrias A., Ennas G., Musinu A., “The Sol-Gel Method: Choice of the experimental conditions”, Materials Science Foundations, Vol.13 |
| [24] | Meera Basa, “Synthesis & Characterization of Silica Coated Iron oxide Nanoparticles by Sol-Gel Technique” M.Sc. Thesis, National institute of Technology, Rourkela 2007. |
| [25] | Lenza. R.F.S., Vasconcelos W.L. “FT-IR of Silicon (IV) oxide” Mat. Res., vol. 4(3), 2001. |
| [26] | Tabatabaei,S., Shukohfar, A., Aghababazadeh, R., Mirhabibi, A., “Experimental study of the synthesis and characterisation of silica nanoparticles via the sol-gel method” Journal of Physics: Conference Series 26, p 371–374,2006. |
| [27] | Raileanu, M., Crisan, Petrache C., Crisan D., Zaharescu M. “Fe2O3-SiO2 Nanocomposites obtained by different sol-gel routes” Journal of Optoelectronics and Advanced Materials Vol. 5, No. 3, p. 693 – 698 ,2003. |
| [28] | Qingyin Wu, “Preparation and proton-conductibility of silica gel containing 64 wt.% undecatungstocobaltoaluminic heteropoly acid”, Materials Letters, vol. 56 , p19– 23,2002. |
| [29] | Balek,V.,Subrt,J., “ Thermal behaviour of iron (III) oxy hydroxide”, Pure & App. Chem., Vol. 67, No. 11, p 1839-1842, 1995. |
| [30] | Guido Ennas, Maria F. Casula, SergioMarras, Gabriele Navarra, Alessandra Scano, and GiaimeMarongiu “Characterisation of FeOOH Nanoparticles and Amorphous Silica Matrix in an FeOOH-SiO2 Nanocomposite” Journal of Nanomaterials Vol.2008. |
| [31] | Palomares, S.A., Anchez, S., Ponce-Castaneda, S. , Mart_ınez, J.R., Facundo Ruiz, Yurii Chumakov, Dom_ınguez, O. , “Quantitative analysis of iron oxide particles Embedded in an amorphous xerogel matrix” Journal of Non-Crystalline Solids,vol.325,p251– 257, 2003. |
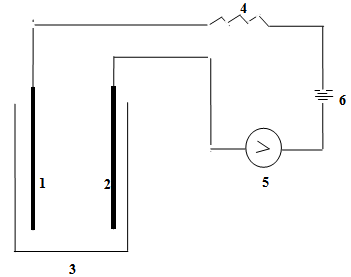
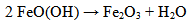

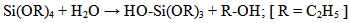 A simple one step protocol was used for the preparation of silica, which involves with the hydrolysis and condensation of tetraethylorthosilicate (TEOS) in ethanol water mixture under acidic condition at room temperature. TEOS, water and ethanol were mixed at 1.00: 10.20: 3.85 molar ratio followed by 9.00 mL water containing 0.20 mL molar HCl [23]. It was then stirred constantly using a thermostated magnetic stirrer at ambient temperature for about one hour. The stirred mixture was then allowed to stand for two days for ageing so that gel formation was completed.The gel of silicon (IV) oxide was collected as a residue by filtration. Distilled water was poured on the silicon (IV) oxide gel several times to ensure removal of chloride or any other undesirable ions from silicon (IV) oxide because the presence of impurity will cover up the surface and hence the actual surface area will decrease. The gel was dried in an oven at 110℃ for 4 hours. Silicon (IV) oxide thus prepared was preserved in a desiccator for characterization. Schematic representation of the process is shown below:
A simple one step protocol was used for the preparation of silica, which involves with the hydrolysis and condensation of tetraethylorthosilicate (TEOS) in ethanol water mixture under acidic condition at room temperature. TEOS, water and ethanol were mixed at 1.00: 10.20: 3.85 molar ratio followed by 9.00 mL water containing 0.20 mL molar HCl [23]. It was then stirred constantly using a thermostated magnetic stirrer at ambient temperature for about one hour. The stirred mixture was then allowed to stand for two days for ageing so that gel formation was completed.The gel of silicon (IV) oxide was collected as a residue by filtration. Distilled water was poured on the silicon (IV) oxide gel several times to ensure removal of chloride or any other undesirable ions from silicon (IV) oxide because the presence of impurity will cover up the surface and hence the actual surface area will decrease. The gel was dried in an oven at 110℃ for 4 hours. Silicon (IV) oxide thus prepared was preserved in a desiccator for characterization. Schematic representation of the process is shown below: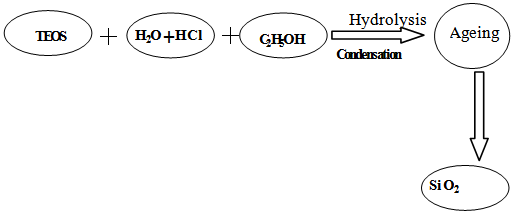
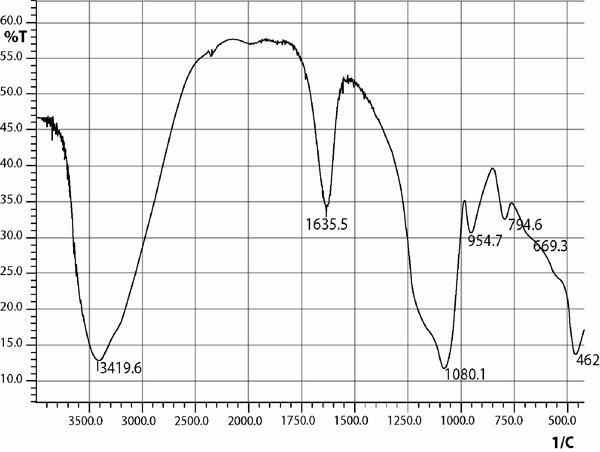
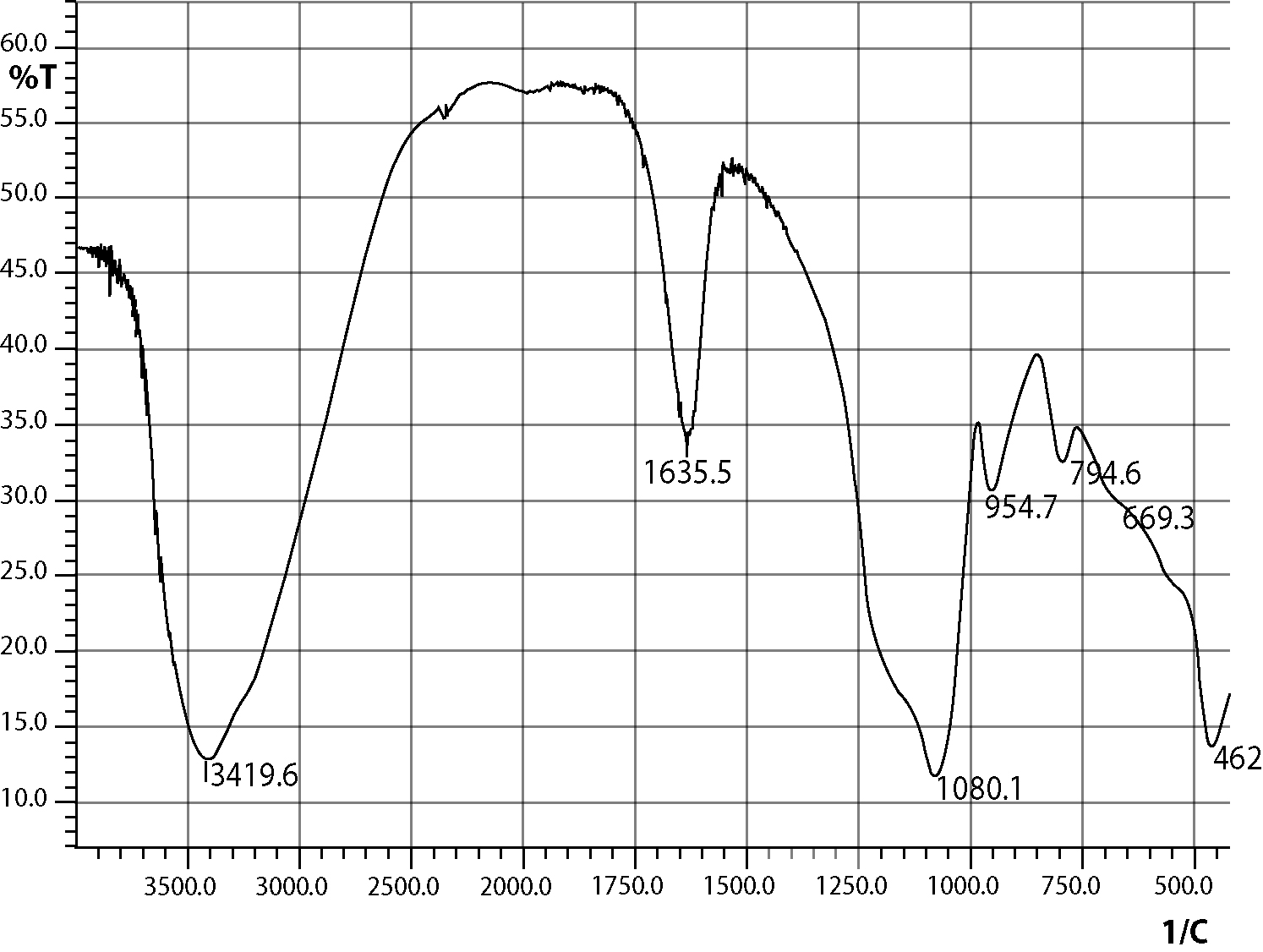
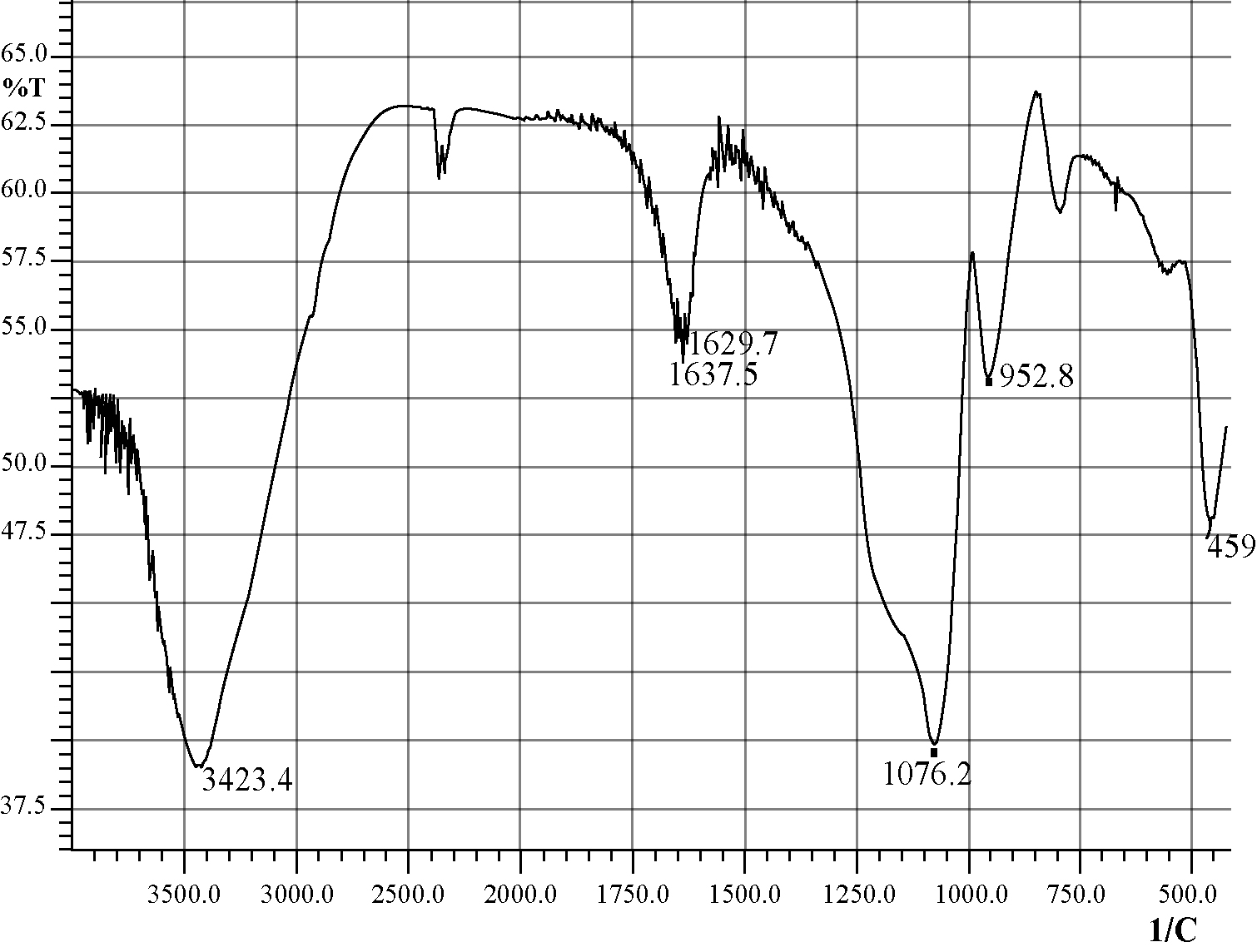
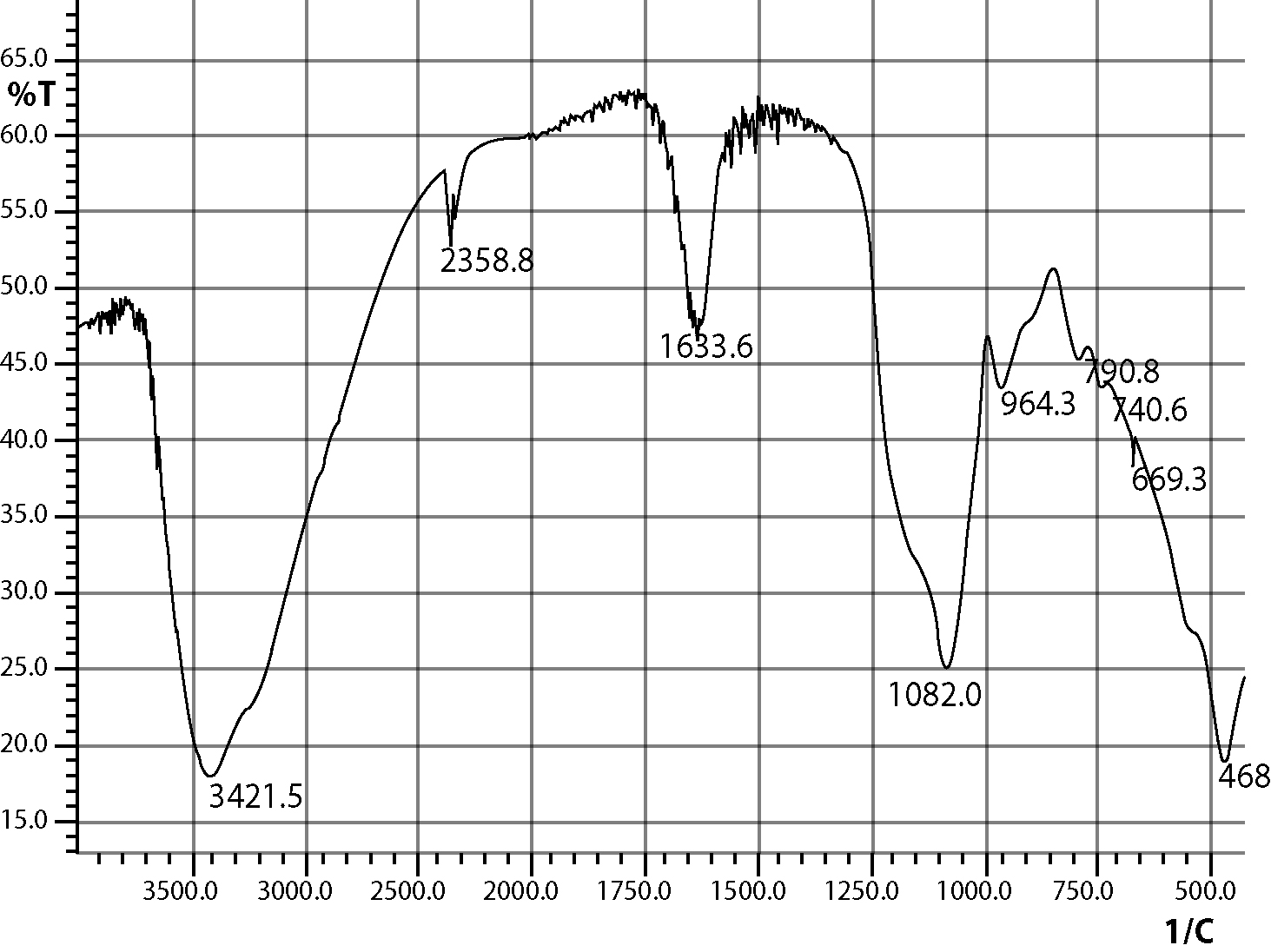
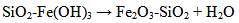 The silica coated iron-oxide composite thus prepared was preserved for characterization and subsequent uses.All three samples of the composite of different ratios were prepared in the same process (only the molar ratio were maintained as 3:7, 1:1 and 7:3).
The silica coated iron-oxide composite thus prepared was preserved for characterization and subsequent uses.All three samples of the composite of different ratios were prepared in the same process (only the molar ratio were maintained as 3:7, 1:1 and 7:3).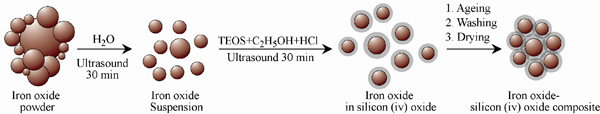
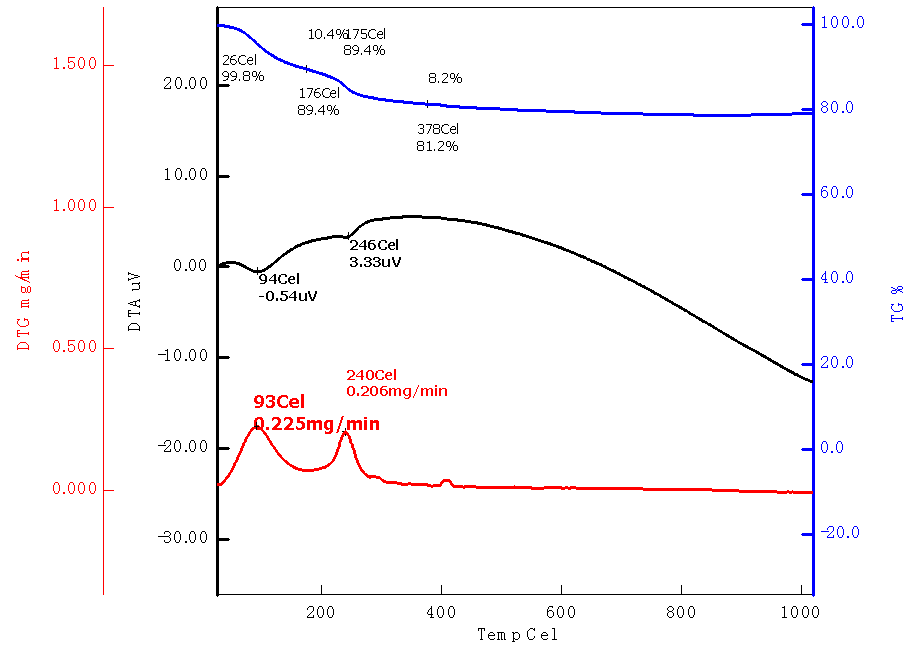
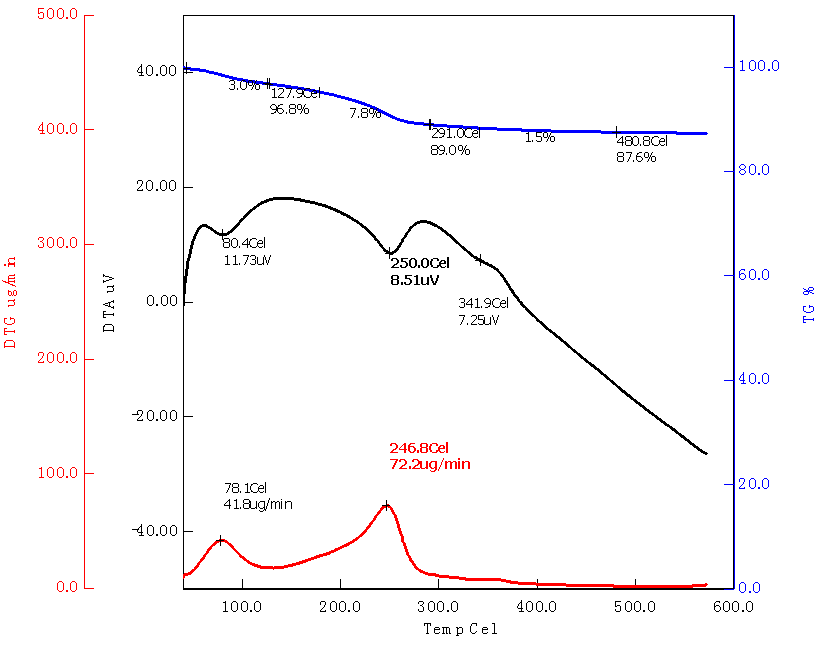
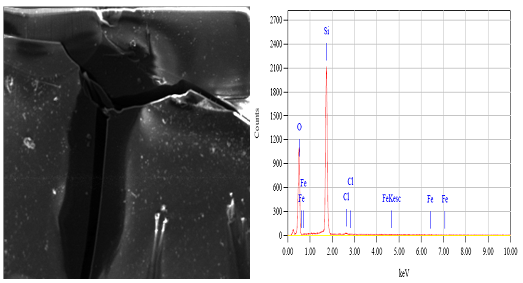
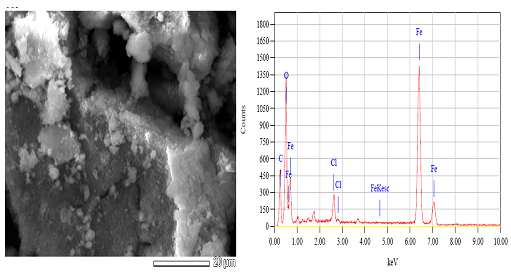
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML