-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2014; 4(1): 4-13
doi:10.5923/j.cmaterials.20140401.02
Transport of n-Hexane through Carbonized Oil Palm Empty  Fruit Bunch Powder Filled Polypropylene/Natural Rubber Biocomposites
Fruit Bunch Powder Filled Polypropylene/Natural Rubber Biocomposites
Chinomso M. Ewulonu1, Godwin N. Onyeagoro2, Iheoma C. Chukwujike1
1Department of Polymer and Textile Engineering, Nnamdi Azikiwe University, Awka, P.M.B. 5055, Awka, Nigeria
2Department of Polymer and Textile Engineering, Federal University of Technology, Owerri, P.M.B. 1525, Owerri, Nigeria
Correspondence to: Chinomso M. Ewulonu, Department of Polymer and Textile Engineering, Nnamdi Azikiwe University, Awka, P.M.B. 5055, Awka, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The transport behaviour of n-hexane through carbonized oil palm empty fruit bunch powder filled polypropylene/natural rubber has been studied at three different temperatures (313, 333, and 353 K) by gravimetric test method. The effect of MAPP as compatibilizer on the sorption properties of the thermoplastic elastomer biocomposites was also investigated. Diffusion, sorption, and permeation coefficients of the biocomposites showed that the uncompatibilized blends sorbed more n-hexane than the compatibilized blends. Equilibrium sorption was found to decrease with increasing filler loading for both compatibilized and uncompatibilized blends due to the fillers’ reinforcing ability. The calculated enthalpies of sorption (ΔHs), Gibbs free energies of sorption (ΔGs) and Arrhenius activation energies (ED and EP) were all positive. This indicates the non-spontaneity of the solubility of carbonized oil palm empty fruit bunch powder filled polypropylene/natural rubber in n-hexane solvent at 313 K. The mode of transport of n-hexane into filled polypropylene/natural rubber was also found to be Fickian and dominated by Henry’s model with endothermic contributions.
Keywords: Transport mechanism, n-hexane, Compatibilizer, Polymer blends, Biocomposites
Cite this paper: Chinomso M. Ewulonu, Godwin N. Onyeagoro, Iheoma C. Chukwujike, Transport of n-Hexane through Carbonized Oil Palm Empty  Fruit Bunch Powder Filled Polypropylene/Natural Rubber Biocomposites, International Journal of Composite Materials, Vol. 4 No. 1, 2014, pp. 4-13. doi: 10.5923/j.cmaterials.20140401.02.
Fruit Bunch Powder Filled Polypropylene/Natural Rubber Biocomposites, International Journal of Composite Materials, Vol. 4 No. 1, 2014, pp. 4-13. doi: 10.5923/j.cmaterials.20140401.02.
Article Outline
1. Introduction
- The study of diffusion, sorption and permeation in polymer blend structures provides a valuable means for additional characterization of polymer blends. These processes are the limiting factors affecting polymer end-use application because these processes may lead to destruction in polymer properties. The diffusion processes in polymer are normally affected by the following factors: the degree of solvent-polymer interactions and solvent properties such as hydrogen bonding, polarity, solubility parameter, nature of additives/fillers among others. The diffusion and transport through polymer blends depend upon its composition, miscibility and phase morphology. The blends may be either homogenous or heterogeneous. In homogeneous blends, the diffusion process is influenced by the interaction between the component polymers[1-3], while in heterogeneous blends interfacial phenomena and the rubbery or glassy nature of the phases are important[4]. The understanding of the transport mechanism of solvents through polymeric blends also helps scientists in the selection of polymer materials and processing considerations to minimize sorption level in potential barrier polymers. Thus, the basic transport phenomenon plays a prominent role in many industrial and engineering applications of polymers[5-6].Thermoplastic elastomers (TPEs) are a new class of thermoplastic materials which their properties can be more easily tailored than the block copolymers by simply changing the ratio of the rubber to plastic in the blend. These materials are normally phase separated systems in which one phase is rubbery at room temperature while the other is hard and solid. They possess the elasticity of a rubber and the thermoplasticity of a plastic, yet retain unique features of its components such as better ultraviolet and ozone resistance, solvent resistance and high deformation temperature compared to elastomers[7]. As a result, many commercial TPEs have been developed for various applications in the automotive, electrical and medical industries[8]. The field of TPEs has grown up along two distinctly different product types. The first type consists of a simple blend of the components and in the second type, the rubber phase is dynamically vulcanized which gives rise to a thermoplastic vulcanizate[9]. Many literature sources have revealed excellent report on the diffusion and sorption processes in elastomer/thermoplastic blends. Thus, transport studies have been conducted on blends of polypropylene/nitrile rubber [10], polystyrene/natural rubber[11].Obasi et al.,[12] investigated the diffusion characteristic of toluene into natural rubber/linearlow density polyethylene with the effects of blend ratio on the diffusion, sorption, and permeation coefficients. The 75/25 NR/LLDPE blend exhibited the highest amount of molar percentage uptake of toluene at the temperatures studied. The diffusion co-efficient, and permeation obtained for toluene in 50/50 and 60/40 NR/LLDPE blends were found to increase with an increase in sorption temperature. The transport of toluene through most of the blends was anomalous, although at 35 oC, the transport of toluene through the 60/40 blend was Fickian and at 55oC, pseudo-Fickian. The effects of fillers on the transport characteristics of TPEs have immerse interest to scientist, and report on the effect of fillers on the diffusion and sorption processes exist[13,14]. Fillers are materials that are added to a polymer formulation to lower the compound cost or to improve its properties. Such materials have reinforcing power which enhances the modulus and any failure property. Example of such fillers are the natural fibres e.g. kenaf, jute, coconut shell, oil palm, corn hubs which are eco-friendly[15], carbon black, clay etc. The major disadvantage of incorporating these fillers into a synthetic polymer is their incompatibility[16,17]. Natural fibres are hydrophilic in nature whereas synthetic polymers are hydrophobic. The hydrophilic characteristics of natural fibres leads toa high moisture absorption, causing dimensional change in the fibre and resulting in the swelling of manufactured composites[18]. Bonstran and Danenberg[19] presented the equilibrium swelling data of filled natural rubber and a number of synthetic rubbers in a variety of solvent. They observed that fillers like carbon black caused a reduction in swelling of the membrane and which commensurate with the volume loading in filler. Investigation on the swelling properties of filled natural rubber/linear low-density polyethylene blends in toluene has been carried out[20]. It was found that the swelling index decreased with increase in filler loading and this was attributed to the increase in the cross – link density of the blend. Studies on the molecular transport of aromatic solvents through microcomposites of natural rubber (NR), carboxylated styrene butadiene rubber (XSBR) and their blends have been carried out[21]. They concluded that the filled samples showed reduced swelling rate owing to the tortuosity of the path. A dramatic decrease in diffusion coefficient of filled samples was also observed. However, blend system exhibited unexpected diffusion behaviour because of the immiscibility of the two components; the activation energy needed for diffusion of penetrant was found to be higher for filled virgin polymers.Furthermore, the effects of filler loading on properties of polypropylene–natural rubber–recycle rubber powder (PP–NR–RRP) composites have been studied[22]. Carbon black, silica and calcium carbonate were used as fillers in the composites. Carbon black filled PP–NR–RRP composites exhibit lowest resistance toward swelling in ASTM oil No.3 than calcium carbonate and silica filled PP–NR–RRP composites. Onyeagoro and Enyiegbulam[23] studied the sorption characteristics of dynamically vulcanized polypropylene/epoxidized natural rubber blend filled with carbonized Dika nutshell. They observed that the mass of toluene sorbed by carbonized dika nutshell filled PP/ENR blends showed an initial increasing trend until maximum absorption is reached, after which equilibrium sorption is reached. Non-vulcanized PP/ENR exhibited the least sorption resistance to toluene. Akporhonor et al.[24] studied the equilibrium sorption properties of palm kernel husk and N330 filled natural rubber vulcanizate as a function of filler volume fraction. They observed that unvulcanized rubber dissolved in a suitable solvent while vulcanized rubber can only swell to an extent determined by the crosslinking density. Vulcanizate with higher concentration of palm kernel husk tends to be more soluble in organic solvent than vulcanizate with higher concentration of carbon black.Filler treatment is the process used to alter the surface chemistry, the energy and the degree of interaction at the filler interface. This leads to enhancement of the mechanical properties. Apart from these benefits, the surface treatment of fillers increases the compatibility, dispersibility and processibility. Many studies have been conducted on the physical and chemical methods to improve the adhesion between the fibre and matrix through a modification of then fibre and/polymer matrix[25]. Effect of treated filler has been found to improve interfacial bonding[26]. In the presence of coupling agent, the water uptake was reduced as better interfacial bonding was established. Transport and mechanical properties were also reported to improve by the use of treated fibres[27].In the present paper, blends of natural rubber and polypropylene have been prepared using carbonized oil palm empty fruit bunch (OPEFB) powderas filler. The diffusion of n-hexane, an aliphatic solvent, through the blends has been investigated and the mechanism of sorption through the blends was determined. The diffusion, sorption and permeation coefficients were calculated. Also, the effects of maleic anhydride-graft-polypropylene (MAPP)compatibilizer and temperature on the diffusion and sorption processes of the thermoplastic elastomer biocomposites were studied.The analysis of diffusion of n-hexane through carbonized OPEFB powder filled natural rubber/polypropylene thermoplastic elastomer biocomposites has not been reported in the scientific literature to our knowledge. The present study affords a very good example of how compatibilizers affect the diffusion of solvents into polymeric blends. The effects of filler loading on the solvent diffusion parameters are also reported.
2. Experimental
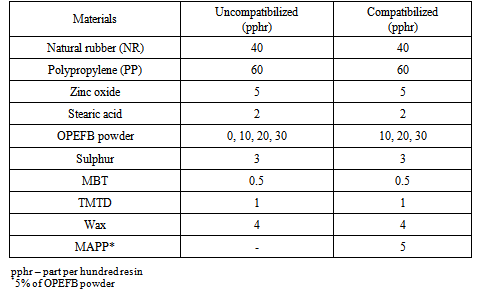
2.1. Materials
- The polypropylene used in this study was purchased from Rovet chemicals limited, Benin-city, Nigeria. It has a melt flow index of 3.5 dg/min, density of 0.905 g/cm3 and specific gravity of 0.905.Natural rubber conforming to the Nigeria Standard Rubber Grade 10 (NSR10) of density 0.92 g/cm3 and specific gravity of 0.92 was used in this study. It was purchased from Iyayi Rubber Factory, Egba, Benin City while other vulcanizing ingredients used such as Zinc Oxide (ZnO), stearic acid, sulphur, MBT(mercaptobenzoylthiazole), TMTD (tetramethylthiuram disulphide) and wax were purchased from a chemical store in Port Harcourt, Nigeria.Oil palm empty fruit bunch (OPEFB) powder was used as filler in the blending of the polypropylene/natural rubber composites. The OPEFB which was collected from Adapalm Nigeria limited, Ohaji, Imo State, Nigeria, is among the waste produced in the processing of palm oil. The oil palm empty fruit bunch was prepared as stated by Ewulonu and Igwe[28]. The prepared fibre was then carbonized at 400oC and sieved through a mesh size of 0.15 mm. The fine powder was collected and used for the blending. Maleic anhydride-graft-polypropylene (MAPP) used as the compatibilizing agent in this study is a product of Sigma-Aldrich Cheme GmbH, Germany and was used as received.
2.2. Preparation of the Thermoplastic Elastomer Biocomposites
|
2.3. Procedure for Sorption Test
- Blends of uniform size were cut and weighed on an analytical balance having an accuracy of 0.001 g. The cut samples were put into sample bottles with covers. 20 ml of n-hexane was poured into each of the sample bottles. The bottles were then placed in a thermostatically controlled water bath at 313 K and were equilibrated for different time intervals. At the expiration of the specified time, the blends were removed from the sample bottles, wiped free of adhering n-hexane, weighed using the analytical balance and the difference between the dry film and the wet film was obtained. The following time intervals were investigated: 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, 180 minutes. Care was taken to ensure that the solvent molecules absorbed by the film were not removed during the process of wiping using filter paper. The experiments were further repeated at 333 and 353K. Each weighing was completed in less than 40 seconds, so as to keep the error due to solvent evaporation from the sample surface at a minimum[29].
3. Results and Discussion
- The uptake of n-hexane by compatibilized and uncompatibilized oil palm empty fruit bunch powder-filled polypropylene/natural rubber vulcanizates have been investigated by gravimetric test method. The sorption data obtained for the filled thermoplastic elastomers at the different temperatures (313, 333, and 353 K) investigated were expressed as the molar percentage uptake (% Qt) of n-hexane per gram of PP/NR/OPEFB biocomposites. % Qt was calculated using the following equation[30,31]
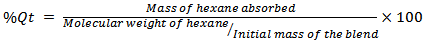 | (1) |
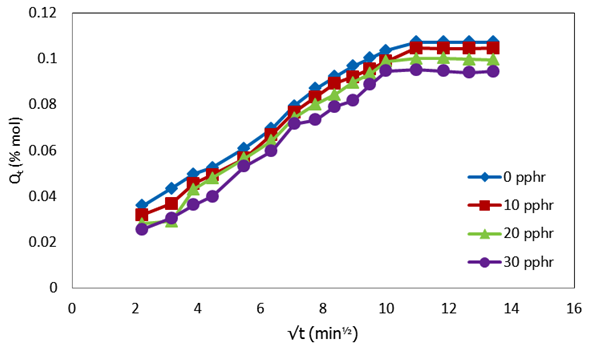 | Figure 1. Plot of molar percentage uptake (% Qt) versus square root of time (√t, min1/2) for uncompatibilized PP/NR blends filled with different levels of carbnized OPEFB powder at 313 K |
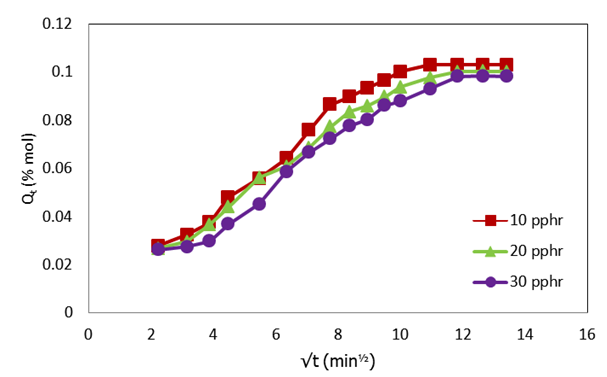 | Figure 2. Plot of molar percentage uptake (% Qt) versus square root of time (√t, min1/2) for compatibilized PP/NR blends filled with different levels of carbnized OPEFB powder at 313 K |
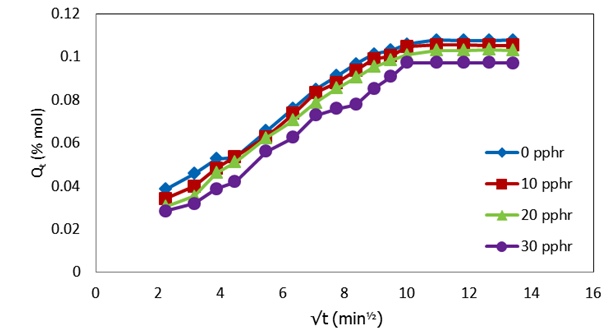 | Figure 3. Plot of molar percentage uptake (% Qt) versus square root of time (√t, min1/2) for uncompatibilized PP/NR blends filled with different levels of carbnized OPEFB powder at 333 K |
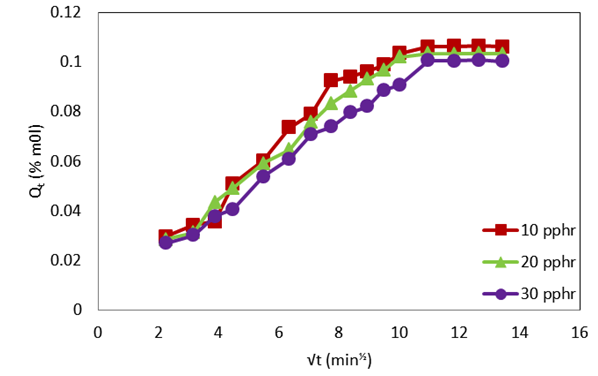 | Figure 4. Plot of molar percentage uptake (% Qt) versus square root of time (√t, min1/2) for compatibilized PP/NR blends filled with different levels of carbnized OPEFB powder at 333 K |
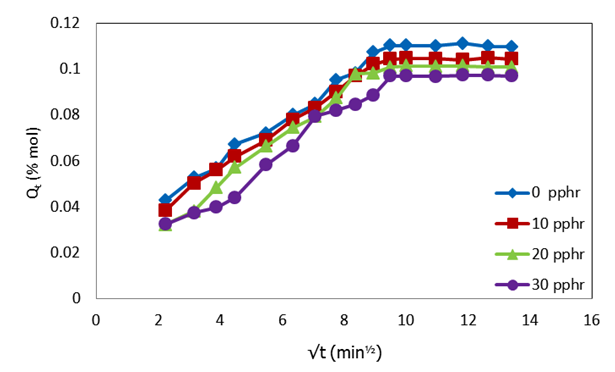 | Figure 5. Plot of molar percentage uptake (% Qt) versus square root of time (√t, min1/2) for uncompatibilized PP/NR blends filled with different levels of carbnized OPEFB powder at 353 K |
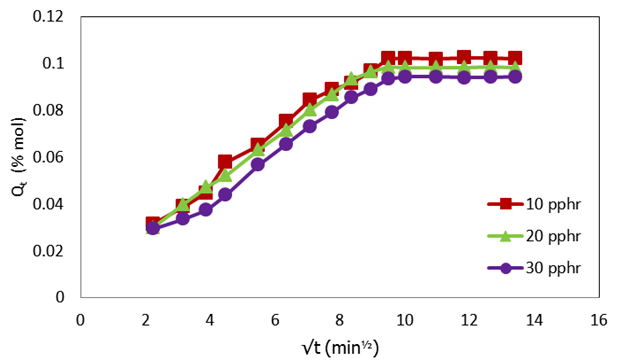 | Figure 6. Plot of molar percentage uptake (% Qt) versus square root of time (√t, min1/2) for compatibilized PP/NR blends filled with different levels of carbnized OPEFB powder at 353 K |
3.1. Diffusion Coefficient (D)
- The diffusion coefficient of a solvent molecule through a polymer membrane can be obtained using Fickian’s second law of diffusion[31].
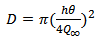 | (2) |
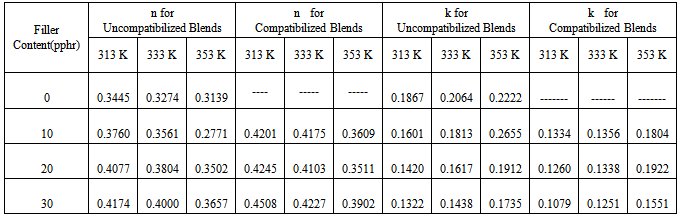
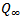 is the equilibrium absorption. The diffusion coefficient values (D) are given in Table 2 along with other sorption parameters. From Table 2, it is evident that the D values for the uncompartibilized blends decreased with increase in filler concentration. Contrarily, the compatibilized blends showed an increase in the diffusion coefficient as the filler content increased for all temperatures studied. This can be attributed to the increased heterogeneity occasioned by the increasing filler content in the compatibilized blends. Note that diffusion process is influenced by interfacial phenomenon and the rubbery or glassy nature of phases for heterogeneous blends[4] and the diffusion and transport through polymer blends depend upon its composition, miscibility and phase morphology. However, it is significant to note that the values of diffusion coefficient for the compatibilized blends are much lower than that of the uncompatibilized blends. For the temperatures studied, the D values for both compatibilized and uncompatibilized blends were found to have the least value at 333K. The same order was not observed for 30 pphr uncompatibilized, where D increased with increases in temperature. This is an indication that there was no strong dependence of D values on temperature at any filler concentration. This is in agreement with the findings of Obasi et al.[12], who reported that no dependence of diffusion coefficient, D on temperature for the amount of NR and LLDPE in their blends was observed. Contrary to this, Igwe et al.[34] found that the diffusion coefficients of solvents (benzene, toluene, and xylene) into polypropylene film increased with increases in sorption temperature. While Igwe and Ezeani,[35] reported that D values generally decrease with increase in sorption temperature of snail shell powder filled natural rubber for any particular solvent and filler particle size. It is important to note that diffusivity in a given polymer system whether it is rubbery or glassy polymers, polymer blends, graft polymers, polymer composites or thermoplastic elastomers varies from one polymer system to another.
is the equilibrium absorption. The diffusion coefficient values (D) are given in Table 2 along with other sorption parameters. From Table 2, it is evident that the D values for the uncompartibilized blends decreased with increase in filler concentration. Contrarily, the compatibilized blends showed an increase in the diffusion coefficient as the filler content increased for all temperatures studied. This can be attributed to the increased heterogeneity occasioned by the increasing filler content in the compatibilized blends. Note that diffusion process is influenced by interfacial phenomenon and the rubbery or glassy nature of phases for heterogeneous blends[4] and the diffusion and transport through polymer blends depend upon its composition, miscibility and phase morphology. However, it is significant to note that the values of diffusion coefficient for the compatibilized blends are much lower than that of the uncompatibilized blends. For the temperatures studied, the D values for both compatibilized and uncompatibilized blends were found to have the least value at 333K. The same order was not observed for 30 pphr uncompatibilized, where D increased with increases in temperature. This is an indication that there was no strong dependence of D values on temperature at any filler concentration. This is in agreement with the findings of Obasi et al.[12], who reported that no dependence of diffusion coefficient, D on temperature for the amount of NR and LLDPE in their blends was observed. Contrary to this, Igwe et al.[34] found that the diffusion coefficients of solvents (benzene, toluene, and xylene) into polypropylene film increased with increases in sorption temperature. While Igwe and Ezeani,[35] reported that D values generally decrease with increase in sorption temperature of snail shell powder filled natural rubber for any particular solvent and filler particle size. It is important to note that diffusivity in a given polymer system whether it is rubbery or glassy polymers, polymer blends, graft polymers, polymer composites or thermoplastic elastomers varies from one polymer system to another.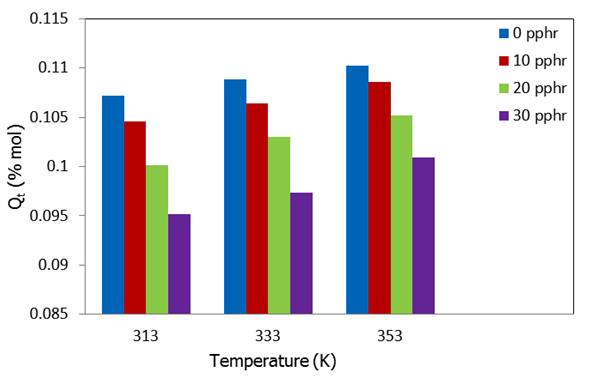 | Figure 7. Dependence of equilibrium n-hexane uptake Qt(% mol) on sorption temperature (K) for uncompatibilized PP/NR blends filled with different levels of carbonized OPEFB powder |
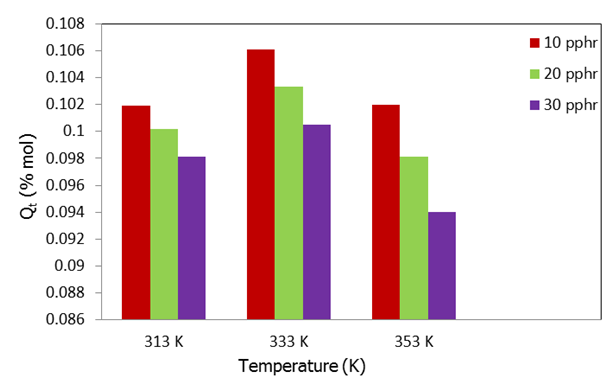 | Figure 8. Dependence of equilibrium n-hexane uptake Qt(% mol) on sorption temperature (K) for compatibilized PP/NR blends filled with different levels of carbonized OPEFB powder |
3.2. Sorption Coefficient (S)
- The sorption coefficient (S) of the carbonized oil palm empty fruit bunch powder filled PP/NR blends was evaluated using Equation 3[31].
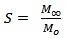 | (3) |
 | (4) |
|
|
3.3. Permeability Coefficient (P)
- The permeability coefficient of n-hexane in the carbonized oil palm empty fruit bunch powder filled PP/NR biocomposite was obtained as follows[31]:
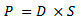 | (5) |
3.4. Transport Mechanism
- The mechanism of diffusion of n-hexane into carbonized OPEFB powder filled PP/NR was analysed by fitting the sorption data into the following empirical relation[36]:
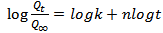 | (6) |
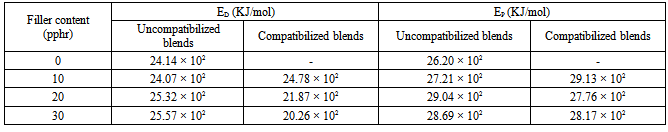
3.4.1. Activation Energy Parameters
- The temperature dependence of transport properties was used to evaluate the activation energy for the diffusion, and permeation processes using the Arrhenius relation[37]:
 | (7) |
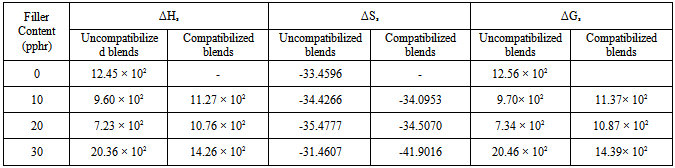
3.4.2. Enthalpy of Absorption (ΔHs) and Entropy of Absorption (ΔSs)
- The enthalpy of absorption (ΔHs) and entropy of absorption (ΔSs) in the MAPP compatibilized and uncompatibilized biocomposites were calculated from the sorption data by first determining the values of the equilibrium absorption constant (Ks) of n-hexane using the following formula[38]:
 | (8) |
 | (9) |
|
3.4.3. Gibbs Free Energy of Sorption (ΔGs)
- The change in ΔGsfor the n-hexane in carbonized OPEFB filled polypropylene/natural rubber blends were obtained using the following expression:
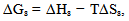 | (10) |
4. Conclusions
- The transport behaviour of n-hexane through carbonized oil palm empty fruit bunch powder filled polypropylene / natural rubber has been studied at three different temperatures (313, 333, and 353 K) by gravimetric test method. The effect of MAPP as compatibilizer on the sorption properties of the thermoplastic elastomer biocomposites was also investigated. Diffusion, sorption, and permeation coefficients of the biocomposites showed that the uncompatibilized blends sorbed more n-hexane than the compatibilized blends. Equilibrium sorption was found to decrease with increasing filler loading for both compatibilized and uncompatibilized blends due to the fillers’ reinforcing ability. The calculated enthalpies of sorption (ΔHs), and Gibbs free energies of sorption (ΔGs) were all positive also the Arrhenius activation energies (ED and EP) were all positive. The positive ΔGs obtained in this study is an indication of non-spontaneity of the solubility of carbonized oil palm empty fruit bunch powder filled polypropylene/natural rubber in n-hexane solvent at 313 K. The mode of transport of n-hexane into filled polypropylene/natural rubber has been found to be Fickian and dominated by Henry’s model with endothermic contributions. The transport parameters presented in this study have not only provided additional characterization of the oil palm empty fruit bunch powder filled polypropylene/natural rubber blends but gave an insight into the behaviour of the thermoplastic elastomer biocomposites in external liquid environment which is essential for their successful applications. The data obtained could be of importance in problem solving like designing a barrier material, gaskets or tubes for transporting liquids.
References
| [1] | Hopfenberg, H. B., Paul, D. R.: Polymer Blends. (eds. Paul D. R.. and Newmann S.) Academic Press, London, (1978). |
| [2] | Paul D. R.: Gas transport in homogeneous multicomponent polymers. Journal of Membrane Science, 18, 75-86 (1984). DOI: 10.1016/S0376-7388(00)85026-7 |
| [3] | Chiou J. S., Paul D. R.: Sorption and transport of CO2 in PVF2/PMMA blends. Journal of Applied Polymer Science, 32(1), 2897-2918 (1986). DOI:10.1002/app.1986. 070320106. |
| [4] | Odani H, Uchikura M, Ogino Y, Kurata M.: Diffusion and solution of methanol vapor in poly(2-vinylpyridine)- block- polyisoprene and poly(2-vinylpyridine)-block-polystyrene. Journal of Membrane Science, 15(2), 193-208 (1983). DOI: 10.1016/S0376-7388(00)80398-1 |
| [5] | Koszinowoski J.: Diffusion and solubility of n-alkanes in polyolefines. Journal of Applied Polymer Science, 32(5), 4765–4786, (1986). DOI: 10.1002/app.1986.070320501 |
| [6] | Berens A. R., Hopfenberg H. B.: Diffusion of organic vapors at low concentrations in glassy PVC, polystyrene, and PMMA. Journal of Membrane Science, 10(2-3), 283–303, (1982). DOI: 10.1016/S0376-7388(00)81415-5 |
| [7] | Zhu S. H., Chan C. M., Zhang Y. X.: Nitrile rubber/natural rubber thermoplastic composites. Journal of Applied Polymer science, 58, 621 (2005). |
| [8] | Cai F., Isayev, N.: Dynamic vulcanization of thermoplastic copolyester elastomer/nitrile rubber alloys: II. rheology, morphology and properties. Journal of Elastomer and Plastics, 25(3), 249-265 (1993). DOI: 10.1177/009524439302500305 |
| [9] | Abdou-sal S., Puydak R. C., Rader C. P.: Dynamic vulcanization of rubber/polyethylene blend. Rubber Chemistry and Technology, 69, 476-482 (2006). |
| [10] | George S. C., Groeninckx G., Ninan K. N., Thomas S.: Molecular transport of aromatic hydrocarbons through nylon-6/ethylene propylene rubber blends: Relationship between phase morphology and transport characteristics. Journal of Polymer Science Part B: Polymer Physics, 38(16), 2136 – 2153(2000).DOI:10.1002/1099-0488(20000815)38:16<2136::AID-POLB70>3.0.CO;2-Z. |
| [11] | Aseletha R., Kumaran M. G., Thomas S.: Transport behaviour of aliphatic hydrocarbon through dynamically cross-linked natural rubber/polystyrene blends. Polymers and Polymer Composites, 6 (6), 357 – 372 (1998). |
| [12] | Obasi H. C., Ogbobe O., Igwe I. O.: Diffusion characteristics of toluene into natural rubber/linear low density polyethylene blends. International Journal of Polymer Science, 54(4), 765-771 (2009). DOI: 10.1155/2009/140682. |
| [13] | Candia F., Gargani L., Renzulli A.: Transport properties of filled elastomeric networks. Journal of AppliedPolymerScience, 41(5-6), 955–964 (1990).DOI:10.1002/app.1990.070410507. |
| [14] | Lawandy S. N., Botros S. H.: Effect of type and amount of carbon black on the interaction between polychloroprene rubber and motor oil. Journal of Applied Polymer Science, 42(1), 137–141 (2001). DOI: 10.1002/app.1991.070420116. |
| [15] | Avella M., Bogoeva-Gaceva G., Gentile G., Grozdanov A.: Poly(lactic acid) based biocomposites reinforced with kenaf fibers. Journal of Applied Polymer Science, 108(6), 3542-3551 (2008). DOI: 10.1002/app.28004. |
| [16] | Lei Y., Wu Q., Yao F., Xu Y.: Preparation and properties of recycled HDPE/natural fiber composites. Composites Part A: Applied Science and Manufacturing, 38(7), 1664-1674 (2007). DOI: 10.1016/j.compositesa.2007.02.001. |
| [17] | [17.]Jarukumjorn K, Suppakarn N.: Effect of Glass Fiber Hybridization on Properties of Sisal Fiber- Polypropylene Composites. Composites Part B: Engineering, 40(7), 623-627 (2009). DOI: 10.1016/j.compositesb.2009.04.007. |
| [18] | Mirbagheri J., Tajvidi M., Hermanson J. C., Ghasemi I.: Tensile properties of wood flour/kenaffiber polypropylene hybrid composites. Journal of Polymer Science, 105(5), 3054-3059 (2007). DOI: 10.1002/app.26363. |
| [19] | Bonstra B. S. T., Dannenberg E. M.: The equilibrium swelling data of filled natural rubber. Rubber Age, 1, 825–838 (1958). |
| [20] | Ahmed A., Mohd D. H., Abdullah I.: Mechanical properties of filled natural rubber/linear low density polyethylene blends. Iranian Polymer Journal, vol. 13(3), 173–178 (2004). |
| [21] | Ranimol S., Kuruvilla J., Zachariah O., Thomas S.: Molecular transport of aromatic solvents through microcomposites of natural rubber/carboxylated styrene butadiene rubber. Journal of Composites Science and Technology, vol. 67(6), 1187-1194 (2007). DOI: 10.1016/j.compscitech.2006.05.009. |
| [22] | Ismail H., Suryadiansyah S.: Effects of filler loading on properties of polypropylene–natural rubber recycle rubber powder (PP–NR–RRP) composites. Journal of Reinforced Plastics and Composites, 23(6), 639 (2004). DOI: 10.1177/ 0731684404032869. |
| [23] | Onyeagoro G. N., Enyiegbulam M. E.: Sorption characteristics of dynamically vulcanized PP/epoxidized NR blends filled with carbonized Dika nutshell. Academic Research Intimation, 3 (2), (2012). |
| [24] | Akporhonor E. E., Egwaihide P. A., Okieimen F. E.: Equilibrium sorption properties of palm kernel husk and N330 filled natural rubber vulcanizate as a function of filler volume fraction. Scientific Research and Essay, 2, (2007). |
| [25] | Liu C. F., Sun R., Qin M., Zin A., Ren J., Xu F., Ye J., Wu S.: Chemical modification of ultrasound pre-treated sugarcane bagasse with maleic anhydride. Industrial Crops and Products, 26(2), 212-219 (2007). DOI: 10.1016/j.indcrop.2007.03.007. |
| [26] | Supri A. G., Lim B. Y.: Effect of treated and untreated filler loading on the mechanical, morphological and water absorption properties of water hyacinth fibers LDPE- composite. Journal of physical science, 20(2), 85-96 (2009). |
| [27] | Behzad K.: Effect of compatibililzer and nanalayered silicate on physical and mechanical properties of PP/Bagasse composite. Tubitak Journal of Agriculture, 36, 510 – 527 (2012). |
| [28] | Ewulonu, C. M., Igwe, I. O.: Properties of oil palm empty fruit bunch fibre filled high density polyethylene. International Journal of Engineering and Technology 3(6), 458 – 471 (2011). |
| [29] | Unnikrishnan, G., Thomas, S.: Diffusion and transport of aromatic hydrocarbons through natural rubber. Polymer. 35(25), 5504 – 5510 (1994). DOI: 10.1016/ S0032-3861 (05) 80015-1. |
| [30] | Mathew A. P., Packirisamy S., Stephen, R., Thomas S.: Transport of aromatic solvents through natural rubber/ polystyrene (NR/PS) interpenetrating polymer network membranes, Journal of Membrane Science, 201(1-2), 213-227 (2002).DOI: 10.1016/S0376-7388(01)00738-4. |
| [31] | Danwanichakul D., Jaroenkarn S., Jumpathi P., Decho-jarassri, D.: Sorption and desorption of toluene, m-xylene, p-cresol and water by natural rubber chips. Songklanakarin Journal of Science and Technology, 28(5), 1071 – 1082 (2006). |
| [32] | George S. C., Thomas S.: Transport phenomena through polymeric systems. Progress in Polymer Science, 26(2001), 985-1017 (2000). DOI: 10.1016/S0079-6700(00)00036-8. |
| [33] | Das B.: Restricted equilibrium swelling—A true measure of adhesion between short fibers and rubber. Journal of Applied Polymer Science, 17(4), 1019-1030 (1973).DOI: 10.1002/ app.1973.070170403. |
| [34] | Igwe I. O., Ewulonu C. M., Igboanugo I.: Studies on the diffusion characteristics of some aromatic solvent into polypropylene films. Journal of Applied Polymer Science, 102(2), 1985 – 1989 (2006). DOI: 10.1002/app.24593. |
| [35] | Igwe I. O., Ezeani O. E.: Studies on the transport of aromatic solvents through filled natural rubber. International Journal of Polymer Science, 2012, 1 – 11. (2012). DOI:10.1155/2012/212507. |
| [36] | Lutch L. M., Peppas N. A.: Transport of penetrants in the macromolecular structure of coals. V. Anomalous transport in pretreated coal particles, Journal of Applied Polymer Science, 33(5), 1557–1566 (1987). DOI:10.1002/app.1987.070330511 |
| [37] | Mathai A. E., Thomas S.: Transport of aromatic hydrocarbons through cross-linked nitrile rubber membranes, Journal of Macromolecular Science Part B, 35(2), 229-253 (1996). DOI: 10.1080/00222349608212383 |
| [38] | Unnikrishnan G., Thomas S., Varghese S.: Sorption and diffusion of aromatic hydrocarbons through filled natural rubber. Polymer, 37(13), 2687–2693 (1996). DOI: 10.1016 /0032-3861(96)87629-4. |
| [39] | Johnson T., Thomas S.: Effect of epoxidation on the transport behaviour and mechanical properties of natural rubber, Polymer, 41(20), 7511–7522 (2000). DOI: 10.1016/S0032- 3861 (00)00076-8. |
| [40] | Igwe I. O.: Uptake of aromatic solvents by polyethylene films. Journal of Applied Polymer Science, 104(6) 3849–3854 (2007). DOI: 10.1002/app.25980. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML