-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2013; 3(4): 100-107
doi:10.5923/j.cmaterials.20130304.03
New Series of Dimethacrylate-Based Monomers on Isosorbide as a Dental Material: Synthesis and Characterization
Zhila Vazifehasl1, 2, Salar Hemmati1, Mohammadreza Zamanloo2, Solmaz Maleki Dizaj3
1Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Applied Chemistry, Faculty of Science, University of Mohaghegh Ardabili, Ardabil, Iran
3Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
Correspondence to: Zhila Vazifehasl, Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Suggestion of new routes in the synthesis of structural units of compounds would be useful in optimization of existing methods, in terms of cost, availability of materials and other problems. Due to some problems in the synthesis of derivatives of D -sorbitol, a few studies have been done in this area. In this study, we offer an effective route for synthesis of a new series of optically and biologically active dimethacrylate-based monomers on isosorbide. Synthesis began with preparation of monobromo derivatives of ethylene glycols and protection their hydroxyl group as tetrahydropyranyl (THP) ethers and then followed by reaction of isosorbide with monobromo derivatives of THP-protected ethylene glycols in the presence of NaH/DMF, deprotection of intermediates in acidic condition to give diol derivatives and finally production of dimethacrylate monomers contain isosorbide skeleton linked to ethylene glycol moieties from diols. The structures of obtained monomers were characterized by Fourier transform infrared (FTIR) and 1H nuclear magnetic resonance (NMR) spectroscopy. Since the dental restorative materials are based on various methacrylate monomers and oligomers, these new obtained monomers are suggested as a dental material.
Keywords: Isosorbide, Ethylene Glycol Derivatives, Protection, Dimethacrylate Monomer, Crosslinking Agent, Dental Composites
Cite this paper: Zhila Vazifehasl, Salar Hemmati, Mohammadreza Zamanloo, Solmaz Maleki Dizaj, New Series of Dimethacrylate-Based Monomers on Isosorbide as a Dental Material: Synthesis and Characterization, International Journal of Composite Materials, Vol. 3 No. 4, 2013, pp. 100-107. doi: 10.5923/j.cmaterials.20130304.03.
Article Outline
1. Introduction
- The monomers are the backbone of polymers and composites. Nowadays, there are a number of methods and procedures for synthesis most-used monomers for developing of present polymers[1-3].Various factors affect the selection of a route or method. For example availability of the initial materials, cost and toxicity of materials, utilization cases and so on.1,4:3,6-Dianhydro-D-glucitol (isosorbide) iscommercially available in large quantities as an important by-product from the starch industry and it is obtained by dehydration of D-glucitol[4-6]. It is thermally stable up to 280°C, of low cost, and available in large quantities[7]. The compound has two hydroxyl groups, one at C-2 having the exo-orientation with respect to the V-shaped molecule, and the other at C-5 having the endo-orientation and involved in intermolecular hydrogen bonding with the oxygen atom of the neighbouring tetrahydrofuran ring[8, 9]. Isosorbide has already been investigated as a starting material for the synthesis of chiral promoters in organic synthesis and notably asymmetric phase transfer catalysis[10, 11]. Isosorbide is a non-toxic compound, and different reactivity of its two hydroxyl groups is a useful strategy to utilize in medical and pharmaceutical applications[10, 12, 13].Developing new routes in synthesis, can be helped to remove or reduce existing problems of current methods. Much less work has been done on derivatives of D-sorbitol, which is explained by the fact that they contain two hydroxyl groups of different nature and are difficult to functionalize in a controlled fashion. It can be expected that further use of these peculiar compounds largely depends on whether effective methods of their controlled functionalization with the aim of design of new structures, includingsupramolecular, will be developed[14]. In order to eliminate these disadvantages, some proposals have been made to modify D-sorbitol derivatives. For this purpose, conversion of hydroxyl groups to acetyls was suggested itself because this grouping is not only stable under alkaline condition, but also is readily cleaved by mild action of aqueous acids with the regeneration of the hydroxyl group[15].Dihydropyranylation of the hydroxyl groups has been recognized as the useful and representative method for the protection of alcohols. To dihydropyranylation of alcohols, p-toluensulfonic acid is the most common catalyst and seems to be superior to other catalysts such as hydrochloric acid, phosphoryl chloride, and boron trifluoride etherate[16-18].Diols play important role in the development of polymeric materials (including, polyesters, polyurethane,polycarbonates, etc.), and today have important applications in the preparation of biological materials[13, 19-21]. including the importance of these compounds in the synthesis of crown ethers, usually using a diol and a two-factor user alkylating has two sides[21-24]. The diol dimethacrylates and glycol dimethacrylates are carried out using two main methods. The first one is transesterification between methyl methacrylate and the higher diols or glycols with constant evaporation of the methanol. The second one is the reaction between methacryloyl chloride and diol or glycol[25, 26].Developing of new routes in the production of monomers, introduces new monomers and therefore new polymers and composites with new properties and perhaps better degree of usefulness. Dimethacrylate monomers are used in a wide variety of cross-linked polymer applications, including adhesives, coatings, dental restoratives, andstereolithography[27-36]. The dental restorative materials are based on various methacrylate monomers and olygomers[36, 37]. Investigations into this group of compounds were begun as early as the 1940s. During these years, methyl methacrylate was used[28-31, 37,38]. Today, new derivatives of methacrylates, acrylates and dimethacrylates havebiomedical applications in bone cements, dental fillings, and hard and soft contact lenses[39]. Another important dental application of radically polymerizable crosslinkers is their use as reactive diluents in filling composites, for example, to dilute the highly viscous bisphenol A diglycidyl dimethacrylate (Bis-GMA), its ethoxylated analog (Bis-EMA) and urethane dimethacrylates (UDMA) along with low molecular weight diluents[40-42].In our previous study, we synthesized the novel diglycidyl methacrylate-based macromonomers from glycolated isosorbide derivatives and glycidyl methacrylate via ring opening reactions[43]. We report here our preliminary investigations on the synthesis of a new series of dimethacrylate-based monomers on isosorbide in dental materials applied as optically active crosslinking agents. To achieve this objective reaction, isosorbide was reacted with monobromo derivatives (mono, di, and tri) of ethylene glycols in which one of the hydroxyl groups is protected, to obtain the relative intermediate. Then, deprotection of intermediates under acidic conditions gave diol derivatives. The resulting diols were reacted with methacryloyl chloride moiety to yield dimethacrylate-based monomers of isosorbide.
2. Experimental
2.1. Material
- Isosorbide(1,4:3,6-Dianhydro-D-glucitol), methacryloyl chloride, mono, di, and tri- ethylene glycols, phosphorus tribromide and p-toluensulfonic acid monohydrate were supplied by Merck (Darmstadt, Germany) and used without further purification. 3,4-Dianhydro-2H-pyran (DHP) was supplied by Merck, dried using Na2CO3 and was distilled (Bp; 84-85°C) before use. Tetrahydrofurane (THF) was supplied by Merck, dried by refluxing over sodium and distilled under argon prior to use. All other reagents were purchased from Merck and purified according to the standard methods.
2.2. Bromination of Ethylene Glycol Derivatives
- Phosphorus tribromide (23 mmol) was added slowly to the stirred solution ethylene glycol (200 mmol) at -5°C; this phase of the reactions was exothermic. Then, the temperature was increased slowly to room temperature and the reaction mixture was refluxed gently for 2 h. The mixture was distilled under reduced pressure to give the monobromo ethylene glycol derivatives (Yield: 79%, 76%, and 72%, for mono (2a), di (2b), and tri (2c) ethylene glycols, respectively).
2.3. Protection of Hydroxyl Group of Monobromo Ethylene glycol Derivetives
- To an ice-cooled solution of monobromo glycol product (40 mmol) and freshly distilled 3,4-dianhydro-2H-pyran (DHP) (47 mmol) in dry THF (50 mL) was added p-toluenesulfonic acid monohydrate (0.68 mmol). The mixture was stirred at 0°C for 30 minutes. Then, the solution was stirred at an ambient temperature for another 12 h and the solvent was removed under vacuum. The residue was worked up with CH2Cl2 and saturated brine. The organic phase was washed with 2×15 mL saturated sodium bicarbonate and 15 mL water and dried (using Na2SO4). Then, the solvent was evaporated and the product was further purified with silica-gel column chromatography using ethyl acetate/n-hexane (30/70 v/v) as the eluent (Yield: 88%, 85%, and 84%, for mono (3a), di (3b), and tri (3c) ethylene glycols bromide, respectively).
2.4. Alkylation of Isosorbide
- In a tree-neck round-bottom flask equipped with condenser, dropping funnel, gas inlet/outlet, and a magnetic stirrer, isosorbide (12 mmol) was dissolved in anhydrous N,N-dimethylformamide (DMF) (50 mL) and added under N2 atmosphere to 25.4 mmol hexane washed NaH (from 60% suspension in oil). The mixture was stirred for about 2 h at 50°C. In a separate container, 25.4 mmol of alkylation agent (THP-protected monobromo ethylene glycols) was dissolved in 10 mL DMF and slowly added to the mixture under N2 atmosphere and refluxed for 24 h at room temperature. The solvent was removed under reduced pressure, and the residue was extracted with CHCl3/H2O mixture (1/1 v/v). The organic layer was dried (using Na2SO4), the solvent was evaporated, and the resulting crude product was further purified on silica-gel column chromatography usingpetroleum ether /n-hexane (80/20 v/v) as the eluent (Yield: 78%, 75%, and 73%, for THP-protected mono (5a), di (5b), and tri (5c) ethylene glycols bromide, respectively).
2.5. Deprotection of Hydroxyl Group of Alkylated Isosorbide Derivetives
- Alkylated isosorbide (6.6 mmol) was completely dissolved in 12 mL of methanol. Then, 0.3 mL HCl (37%) in 5 mL of methanol was added, and the mixture was stirred at room temperature for 8 h. After the removal of methanol, to neutralize HCl, potassium carbonate (K2CO3) was added. The residue was extracted with chloroform, dried (using Na2SO4) and the solvent was evaporated under reduced pressure to produce a crude product. The obtained diol was further purified with silica-gel column chromatography using ethyl acetate/methanol (90/10 v/v) as the eluent (Yield: 96%, 93%, and 91%, for THP-protected mono (6b), di (6c), and tri (6d) ethylene glycolatedisosorbide, respectively).
2.6. Synthesis of Monomers
- Methacryloyl chloride (9.4 mmol) was dissolved in 5 mL chloroform and was added drop wise to a stirring solution of diol (4.1 mmol) and triethylamine (9.4 mmol) mixture in 35 mL dry chloroform at 0-5°C. The reaction mixture was stirred for 24-36 h at room temperature. The solution was washed successively by the addition of 20 mL water, 2×20 mL NaOH (1 N), and 20 mL water. After drying the organic phases using anhydrous Na2SO4 and evaporation of the solvent, the crude product (dimethacrylate-based monomers of isosorbide) was further purified by column chromatography with ethyl acetate/n-hexane (30/70 v/v) as the eluent (Yield: 77%, 76%, 71%, and 68% for isosorbide (7a), 7b, 7c, and 7d,respectively).
2.7. Characterization
- Fourier transform infrared (FTIR) spectra of the samples were obtained on a Shimadzu 8101M FTIR (Shimadzu, Kyoto, Japan). The spectra were recorded at room temperature. 1H nuclear magnetic resonance (NMR) spectra were obtained at 25°C on an FT-NMR (400MHz) Bruker spectrometer (Bruker, Ettlingen, Germany). The sample for 1H-NMR spectroscopy was prepared by dissolving about 10 mg of products in 5 mL of deuterated chloroform.
3. Results and Discussion
- O-alkylation of glycolic derivatives is based on existence of a leaving group at one end and a protected hydroxyl group at the other end of them. Tosylate or bromo derivatives is often the leaving group of choice as its preparation from the corresponding alcohol takes place under mild conditions, which avoids the stereochemical uncertainties and skeletal rearrangements associated with the conversion of an alcohol to a halide[38].
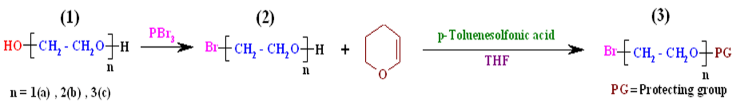 | Scheme 1. Bromination of ethylene glycol derivatives and protection of their hydroxyl groups |
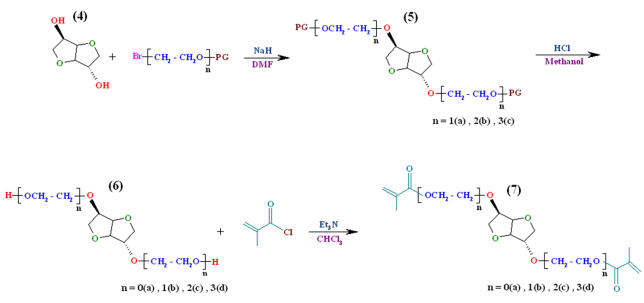 | Scheme 2. Synthesis of alkylated isosorbide derivatives, deprotection of their hydroxyl groups and synthesis of macromonomers |
- Usage of bromo or tosyl derivatives leaving group in self glycol compounds has nearly equal preference, though bromides give better results than tosylate[20]. With regard to this issue, monobromo glycol compounds such asbromoethanol, monobromo diethylene glycol and monobromo triethylene glycol as reactive alkylation agents of isosorbide were chosen. This article consists of five parts: (1) monobromination of ethylene glycol derivatives using PBr3 under free solvent condition; (2) protection of hydroxyl group ofmonobrominated ethylene glycol derivatives with 3,4- dianhydro - 2H - pyran (DHP); (3) O-alkylation of sterically hindered isosorbide by the reaction with the hydroxyl protected ethylene glycols bromide; (4) deprotection of hydroxyl group of alkylated isosorbide derivatives in acidic condition; (5) synthesis and characterization of chiral dimethacrylate - based monomers on isosorbide by reaction of methacryloyl chloride with obtained diols.The methodology followed is shown in Scheme 1 and Scheme 2.
3.1. Bromination of Ethylene Glycol Derivatives and Protection of Their Hydroxyl Group
3.2. Characterization of Alkylated Isosorbide Derivatives
- We have used an efficient O-alkylation of sterically hindered isosorbide by the reaction with the THP-protected ethylene glycol bromide derivatives (3a-c) in the superbasic medium NaH/DMF during 24 h. To get evidence that the obtained alkylation reagents were chemically bonded to the isosorbide (5a-c), FTIR spectroscopy investigation was firstly used to identify the qualitative composition of compounds. The FTIR spectra of1,4:3,6-Dianhydro-D-sorbitol, 2,5-di-O-(2-ethoxy) bis tetrahydro-2H-pyran (5a) (Figure 1a), 1,4:3,6-Dianhydro-D-sorbitol,2,5-di-O-(2-(2΄-ethoxy) ethoxy) bis tetrahydro-2H-pyran (5b) (Figure 2b), and 1,4:3,6-Dianhydro-D-sorbitol,2,5-di-O-(2-(2΄-(2˝-ethoxy) ethoxy) ethoxy) bis tetrahydro-2H-pyran (5c) (Figure 1c) are shown in Figure 1. The characteristic absorption bands due to the stretching vibrations of C-H appear in the 2940-2855 cm-1 region, and C-O-C in the 1050-1250 cm-1 region. The bands around 1453 and 1372 cm-1 are due to the -CH2 groups in the products. Disappearance of the hydroxyl groups of isosorbide in the FTIR spectrum verified the complete conversion of O-alkylation reaction.
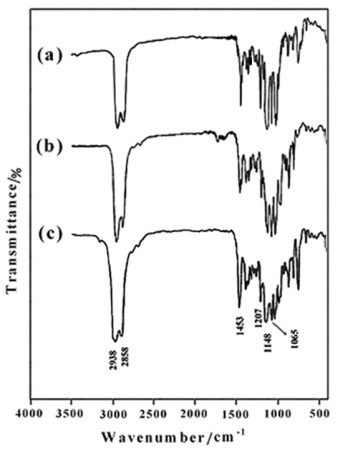 | Figure 1. FTIR spectra of 5a (a), 5b (b) and 5c (c) |
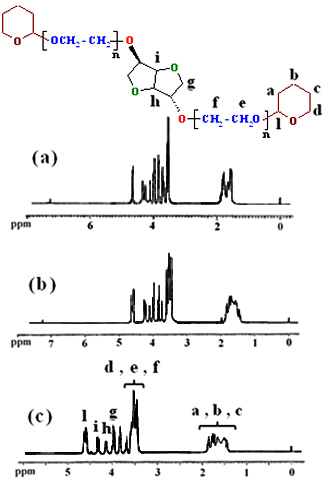 | Figure 2. 1H-NMR spectra of 5a (a), 5b (b) and 5c (c) |
3.3. Deprotection of Hydroxyl Group of Alkylated Isosorbide Derivatives
- The protecting groups (THP) can be removed by reductive method. The obtained dioles were chemically pure and in high yields (91-96%). The FTIR spectra of 1,4:3,6-Dianhydro-D-sorbitol, 2,5-di-O-(2-Hydroxyl ethoxy) (6b) (Figure 3a), 1,4:3,6-Dianhydro-D-sorbitol, 2,5 – di – O - (2-(2΄-Hydroxyl ethoxy) ethoxy) (6c) (Figure 3b), and 1,4:3,6-Dianhydro-D-sorbitol, 2,5-di-O-(2-(2΄-(2˝-Hydroxyl ethoxy) ethoxy) ethoxy) (6d) (Figure 3c) are shown in Figure 3. The most distinctive feature of compounds (6b-d), in comparison with compounds (5a-c), in FTIR spectra is the presence of a broad hydroxyl band at 3410–3420 cm-1.The chemical structures of the obtained diols (6b-d) were further examined by 1H-NMR. The 1H-NMR spectra of the products are shown in Figure 4. The 1H-NMR spectra of the obtained diols indicates that the protecting groups in the compounds (5a-c), were removed by reductive method, because compared with Figure 2 the chemical shifts at 1.40-1.87 and 4.55-4.62 ppm (which is related to -CH2 and O-CH2-O protons of tetrahydropyran, respectively)completely disappears, and the new peak for the hydroxyl groups are observed at 2.64 ppm. These new chiral diols containing isosorbide can be used to obtain the new dimetacrylate - based monomers on isosorbide of interest in asymmetric synthesis.
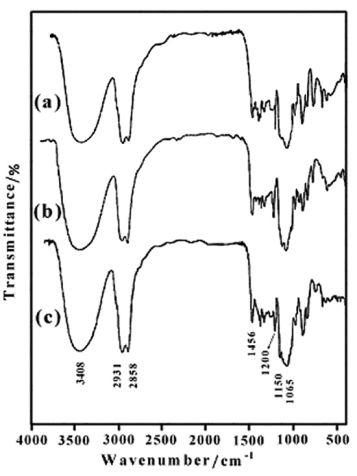 | Figure 3. FTIR spectra of 6b (a), 6c (b) and 6d (c) |
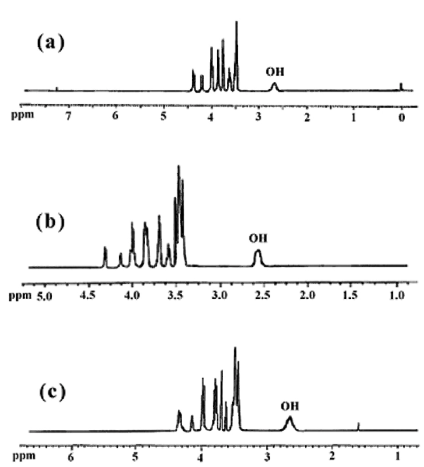 | Figure 4. 1H-NMR spectra of 6b (a), 6c (b) and 6d (c) |
3.4. Synthesis of Monomers
- Monomers are usually referred to as reactive oligomers, polymers or large molecules in which a polymerizable functional group is incorporated into the chain end(s). The use of monomers provides a facile route to control the molecular structure of produces, including crosslinked and branched compounds[44].From the reaction of diols (6b-d) with methacryloyl chloride, novel dimethacrylate-based macromonomers of isosorbide can be synthesized. Due to the high reaction rate of methacryloyl chloride, we choose this reagent as the methacrylated agent. Figure 5 shows the FTIR spectra of 1,4:3,6-Dianhydro-D-sorbitol, 2,5-di-O-bis methacrylate (7a) (Figure 5a), 1,4:3,6-Dianhydro-D-sorbitol, 2,5-di-O- (2-ethoxy) bis methacrylate (7b) (Figure 5b), 1,4:3,6- Dianhydro-D-sorbitol, 2,5-di-O-(2-(2΄-ethoxy) ethoxy) bis methacrylate (7c) (Figure 5c), and 1,4:3,6-Dianhydro- D-sorbitol, 2,5-di-O-(2-(2΄-(2˝-ethoxy) ethoxy) ethoxy) bis methacrylate (7d) (Figure 5d). The FTIR spectra of the compound (7d), shows the characteristics of the functional groups present in the synthetic product. The fingerprint characteristic vibrational bands of methacrylate appear at 1712cm-1ν(C=O) and 1452 cm-1ν(C–O). The characteristic absorption bands due to the stretching vibrations of C-H appear in the 2952-2876 cm-1 region, and C=C stretching vibrations appear at 1643 cm-1. It is clear from Figure 5; all synthetic products show similar absorption bands with minor differences.
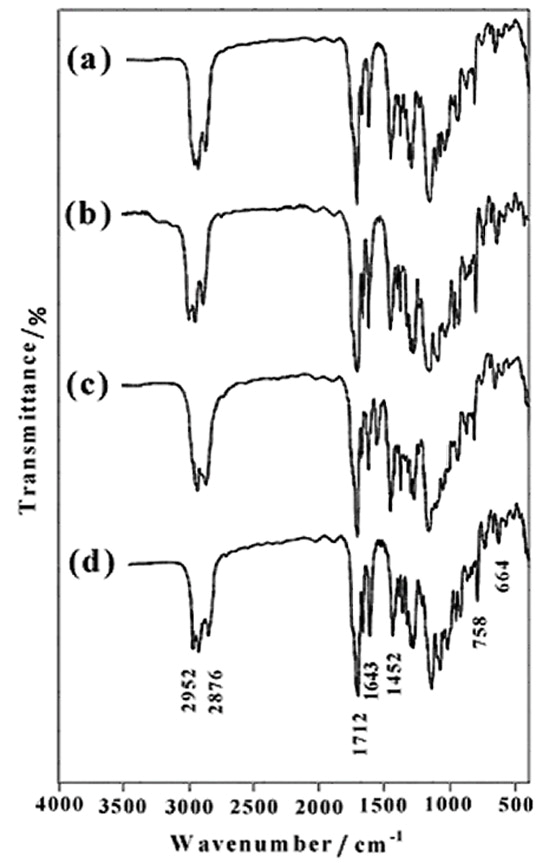 | Figure 5. FTIR spectra of 7a (a), 7b (b), 7c (c) and 7d (d) |
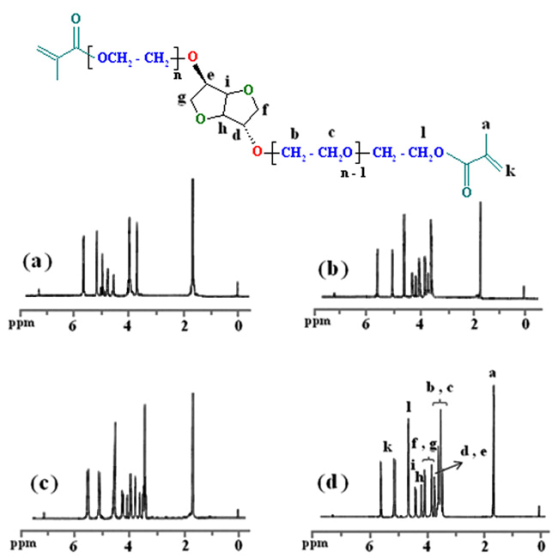 | Figure 6. 1H-NMR spectra of 7a (a), 7b (b), 7c (c) and 7d (d) |
- Additional evidence for the synthesis of thedimethacrylate - based macromonomers of isosorbide was also obtained from 1H-NMR data. The 1H-NMR spectra of monomers indicate the chemical shifts at 1.57-1.71 ppm which is related to the methyl groups (a) of methacrylate and vinyl methylene (k) protons are observed about 5.05-5.23 and 5.54-5.66 ppm. The chemical shifts at 3.35-3.65 ppm, which is related to the –OCH2 protons (b and c) of ethylene glycol segments. The chemical shifts at 3.65-4.10 and 4.20-4.55 ppm shows the -CH2 (f and g) and -CH (h and i) protons of isosorbide, respectively. The chemical shifts of ethereal protons (l) near the carbonyl group due to influence of the anisotropy filed appears at 4.55-4.70 ppm. Furthermore, the 1H-NMR spectra of monomers indicates that all of the hydroxyl groups in the compounds (6b-d), were converted to methacrylate groups because the chemical shift of hydroxyl groups at 2.64 ppm completely disappears.
4. Conclusions
- In summary, a new series of dimethacrylate - based monomers on isosorbide were synthesized andcharacterized. Fourier transform infrared (FTIR) and 1H nuclear magnetic resonance (NMR) spectroscopy studies indicate that dimethacrylate-based monomers on isosorbide were successfully synthesized. In this work, better yields of the complete o-alkylation of isosorbide by reaction in superbasic medium were obtained compared to previously proposed o-alkylaton of aliphatic alcohols. The obtained monomers can be used as diluents in filling composites. Furthermore these monomers are able to react with other methacrylate monomers by radical polymerization and acid-base reaction with the ions leached from the glass used in dental composites. Therefore, due to these properties, they can be suggested as dental material. Soon, in our future work, we will report the synthesis of relative polymers of this new useful monomers.
ACKNOWLEDGEMENTS
- We wish to express our gratitude to the Tabriz University of Medical Sciences and University of Mohaghegh Ardabili, for partial financial supports.
References
| [1] | S. Mallakpour& A. Zadehnazari, eXPRESS Polymer Letters, 5 (2011) 142. |
| [2] | A. Bandyopadhyay& G.M. Odegard, Modelling and Simulation in Materials Science and Engineering, 20 (2012) 45018. |
| [3] | P. Ferreira, J. F. J. Coelho, J. F. Almeida & M. H. Gil, Photocrosslinkable Polymers for Biomedical Applications, InTech publisher, Rijeka, Croatia, 2011, p. 55-74. |
| [4] | S. Chatti, G. Schwarz & H. R. Kricheldorf, Macromolecules, 39 (2006) 9064. |
| [5] | R. Casarano, D. F. S. Petri, M. Jaffe & L. H. Catalani, J. Braz. Chem. Soc. 20 (2009) 1414. |
| [6] | O. N. Van-Buu, A. Aupoix& G. Vo-Thanh, Tetrahedron, 65 (2009) 2260. |
| [7] | D. Abenhaim, A. Loupy, L. Munnier, R. Tamion, F. Marsais& G. Queguiner, Carbohydr Res, 261 (1994) 255. |
| [8] | D. J. Claffey, M. F. Casey & P. A. Finan, Carbohydr Res, 339 (2004) 2433. |
| [9] | M. Yokoe, K. Aoi& M. Okada, J. Appl. Polym. Sci. 98 (2005) 1679. |
| [10] | Y. Zhu, M. Durand, V. Molinier& J. M. Aubry, Green Chem, 10 (2008) 532. |
| [11] | S. Kumar & U. Ramachandran, Tetrahedron, 61 (2005) 4141. |
| [12] | C. Cecutti, Z. Mouloungui& A. Gaset, Bioresource Technol, 66 (1998) 63. |
| [13] | D. Juais, A. F. Naves, Ch. Li, R. A. Gross & L. H. Catalani, Macromolecules, 43 (2010) 10315. |
| [14] | G. I. Kurochkina, G. S. Bratash, N. O. Soboleva, L. K. Vasyanina, M. K. Grachev& E. E. Nifant,ev, Russ. J. Gen. Chem. 74 (2004) 1616. |
| [15] | W. E. Parham & E. L. Anderson, Contribution from the school of Chemistry of The University of Minnesot, 70 (1948) 4187. |
| [16] | N. Miyashita, A. Yoshikoshi& P. A. Grieco, J. Org. Chem. 42 (1977) 3772. |
| [17] | N. Feizi, H. Hassani& M. Hakimi, Bull Korean Chem Soc, 26 (2005) 2087. |
| [18] | Y. T. Reddy, P. N. Reddy, B. S. Kumar, N. Srinivasulu& B. Rajitha, Indian J Chem Sect B, 44 (2005) 2396. |
| [19] | D. K. Chattopadhyay& V. S. N. RajuK, Prog. Polym. Sci. 32 (2007) 352. |
| [20] | M. A. Shaker, J. J. E. Doré& H. M. Younes, J. Biomater. Sci. 21 (2010) 507. |
| [21] | G. Ozer, N. Saracoglu, A. Menzeka& M. Balci, Tetrahedron, 61 (2005) 1545. |
| [22] | E. A. Elperina, R. I. Abylgaziev, M. I. Struchkova& E. P. Serebryakov, Translated from Izvestiya. Akademii. Nack SSSR, Seriya. Khimicheskaya, 3 (1988) 627. |
| [23] | E. A. Elperina, R. I. Abylgaziev& E. P. Serebryakov, Translated from Izvestiya. Akademii. Nack SSSR, Seriya. Khimicheskaya, 3 (1988) 632. |
| [24] | A. Shahrisa& R. Tabrizi, J. Chem. Chem. Eng.18 (1999) 91. |
| [25] | M. Gibas& B. Szapska, Pol. J. Appl. Chem. 93 (1993) 277. |
| [26] | D. Bogdal, J. Pielichowski& A. Boron, J. Appl. Polym. Sci. 66 (1997) 2333. |
| [27] | M. Trujillo-Lemon, J. Ge, H. Lu, J. Tanaka & J. W. Stansbury, J. Polym. Sci. Part A: Polym. Chem. 44 (2006) 3921. |
| [28] | M. Yeli, K. H. Kidiyoor, B. Naik& P. Kumar, Annals and Essences of Dentistry, 2 (2010) 134. |
| [29] | A. Soetojo, Dent. J. (Maj. Ked. Gigi), 40 (2007) 181. |
| [30] | R. Liska, F. Schwager, C. Maier, R. Cano-Vives& J. Stampfl, Journal of Applied Polymer Science, 97(2005) 2286. |
| [31] | A. G. Hervás, M. L. M. Lozano, J. C. Vila, A. B. Escribano& P. F. Galve, Med Oral Patol Oral Cir Bucal, 11(2006) 215. |
| [32] | R. Gauvin, Ch. Y. Ch. Chen, J. W. Lee, P. Soman, P. Zorlutuna, J. W. Nichol, H. Bae, Sh. Chen & A. Khademhosseini, Biomaterials, 33 (2012) 3824. |
| [33] | F. P. W. Melchels, J. Feijen& D.W. Grijpma, Biomaterials, 31(2010) 6121. |
| [34] | P. D. Pham, S. Monge, V. Lapinte, Y. Raoul &J. J. Robin, Eur. J. Lipid Sci. Technol, 115 (2013) 28. |
| [35] | Stuti, J. S. P. Rai& I. Nigam, Malaysian Polymer Journal, 7 (2012) 34. |
| [36] | L. Andreani, L. L. Silva, M. A. Witt, M. M. Meier,A. C. Joussef,&V. Sold, J. Appl. Polym. Sci, 128(2013), 725 |
| [37] | L. Wang, P. H. P. D'Alpino,L. G. Lopes & J. C. Pereira, J. Appl. Oral. Sci. 11 (2003) 162. |
| [38] | F. Kazemi, A. R. Massah& M. Javaherian, Tetrahedron, 63 (2007) 5083. |
| [39] | I. Sideridou, V. Tserki& G. Papanastasiou, Biomaterials, 23 (2002) 1819. |
| [40] | N. Moszner, F. Zeuner, J. Angermann, U. K. Fischer & V. Rheinberger, Macromol Mater Eng, 288 (2003) 621. |
| [41] | C. S. Pfeifer, Z. R. Shelton, R. R. Braga, D. Windmoller, J. C. Machado & J. W. Stansbury, Eur Polym J, 47 (2011) 162. |
| [42] | S. Kammer, K. Keomara, B. Sandner& R. Schreiber, Macromol Mater Eng, 286 (2001) 276. |
| [43] | Zh. Vazifehasl, S. Hemmati, M. Zamanloo, M. Jaymand, Macromol Res, 21(2013), 427. |
| [44] | R. Riva, L. Chafaqi, R. Jérôme, P. Lecomte, ARKIVOK, X (2007) 292 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML