-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2013; 3(3): 61-68
doi:10.5923/j.cmaterials.20130303.04
Cellulose Reinforcement of Phenol Formaldehyde: Characterization and Chemometric Elucidation
Emmanuel Atta-Obeng1, 2, Brian K. Via2, 3, Oladiran Fasina4, Maria L. Auad5, Wei Jiang3, 6
1Forest Products Development Center, Auburn University, Auburn, AL, USA
2Center for Bioenergy and Bioproducts
3School of Forestry and Wildlife Sciences, Auburn University, Auburn, AL, USA
4Department of Biosystems Engineering, Auburn University, Auburn, AL, USA
5Department of Polymer and Fiber Engineering, Auburn University, Auburn, AL, USA
6College of Textiles, Donghua University, Shanghai, China
Correspondence to: Wei Jiang, School of Forestry and Wildlife Sciences, Auburn University, Auburn, AL, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Micro sized crystalline cellulose was uniformly dispersed at loadings of 0, 3, 6, and 10% (by weight) in phenol formaldehyde (PF) and tested for thermal properties and shear strength. The application of cellulose into the PF matrix is considered beneficial because an increase in composite strength will allow for the reduction of petroleum based PF utilized while simultaneously lowering overall formaldehyde concentrations. Characterization studies found this system to exhibit cure temperatures in a very narrow temperature range regardless of cellulose loading which would assist in process changes during manufacturing; however, viscosity increases with cellulose loading was significant and could be a limiting factor.The heat of reaction and nonlinear behavior in lap shear strength with cellulose loading suggests an interaction between cellulose and PF polymer which appeared optimized at 3% cellulose. Finally, utilizing novel chemometric techniques, we were able to partition out the variation in FTIR spectra attributable to a) bulk PF cure and b) cellulose to PF interaction.
Keywords: Cellulose, Phenol Formaldehyde, Chemometric, Infrared
Cite this paper: Emmanuel Atta-Obeng, Brian K. Via, Oladiran Fasina, Maria L. Auad, Wei Jiang, Cellulose Reinforcement of Phenol Formaldehyde: Characterization and Chemometric Elucidation, International Journal of Composite Materials, Vol. 3 No. 3, 2013, pp. 61-68. doi: 10.5923/j.cmaterials.20130303.04.
Article Outline
1. Introduction
- Phenol Formaldehyde (PF) resins has traditionally been utilized in the manufacture of wood composites for structural based applications. PF is utilized for a wide range of applications due to its superior mechanical strength, heat resistance and dimensional stability, as well as, a high resistance against various solvents, acids and water. The most common composite application includes oriented strand board (OSB),plywood, and other engineered wood products, all of which is utilized in residential construction. However, exposure of humans to products in service is common and any formaldehyde emissions that might occur from the adhesive could be of concern, particularly if the resin is not fully cured during processing due to slower cure rates or short press times[1]. Likewise, the reduction of our petroleum based resources is critical if we are to preserve a sustainable resource for future generations.Currently, a primary focus on formaldehyde and phenol reduction through bio-based substitution of the PF matrix has been the most common option[2]. However, the addition of cellulose to the PF matrix is another partial solution to the aforementioned issues.The conversion of biomaterials into cellulose is an established technology utilized by the pulp and paper industry and is readily available in today's market.Furthermore, this source of biopolymer should increase readily as more cellulosic conversion technologies such as thermochemical and fermentation become commercially relevant[3]. Given its crystalline nature, cellulose addition should assist in the increase of composite strength allowing for the reduction in PF polymer loading and consequently lower the utilization of petroleum based phenols and formaldehyde. To date, little research has occurred on this end.Microcrystalline cellulose (MCC) is classified as a polymer with a high aspect ratio and is almost purely crystalline due to the removal of the amorphous regions during acid hydrolysis. While little research has occurred in the addition of cellulose into phenol formaldehyde composites, substitution into other biobased composites has recently been investigated. The addition of MCC into poly lactic acid was tried but the gains in strength were not realized due to the aggregation of cellulose during forming and pelletizing[4]. Thus, other studies have focused on intermediary steps of incorporating organic solvents to assist with homogeneous dispersion. For example, cellulose crystals from MCC were first incorporated into dimethylformamide and sonicated before application to polyurethane composite films[5]. They were also successful in linking the cellulose to matrix by optimization of the isocyanate to crystalline region interaction. It was found that the addition of cellulose also increased the glass transition temperature of the composite and suggests that cellulose could be ideal for high temperature environments. This was supported by others whom add that the addition of cellulose for shear based applications is promising due to the high thermal stability under significant shear conditions such as that observed in PF composite applications[6].For polypropylene-MCC composites, the increase in glass transition temperature was an indication of effective interaction between the matrix and reinforcing elements[7].The mixture of cellulose into PF for wood composite manufacture is perhaps the largest market for such an application and recently has gained importance for wood composites.For example, for particleboard, the addition of cellulose to PF was attempted to increase composite elastic modulus and strength[8].In that case, the high loadings of cellulose, coupled with micron sized cellulosic dimensions, resulted in significant stress at the cellulose to PF interface during composite spring back resulting in the shearing apart of cured PF-cellulose resin (or so it was hypothesized). However, in another study for OSB, this behavior did not occur for microcrystalline cellulose when added to OSB in which the springback did not appear to occur and the strength was increased by 11%[9]. A similar result was found by another recent study which found an increase in strength for both OSB (16%) and particleboard (6%)[10].Because post conversion to nanocellulose is expensive, the small increase in strength that has been witnessed to date may not be justifiable, nor are there many manufacturers that provide nanocellulose in the current market.As a result, micron sized cellulose from pulp and paper processes are probably more feasible and economical. The addition of microcrystalline cellulose to PF adhesives thus appears promising for wood composite manufacture.But first, it would be useful to have a better understanding of the basic interaction mechanism between cellulose and PF resin and the thermal and mechanical properties that could be provided with this greener biocomposite.The interaction between cellulose and PF has been exploredwith FTIR spectroscopy and other characterization tools but to date the findings are conflicting.Wavenumbers associated with the CH2 and CH can be dominated by resin cure during methylation while other studies have found the CH2 and CH functional groups to be a reflection of cellulose to PF polymer interaction[11-13].Because both mechanisms may occur simultaneously, they can interfere with each other and confound the response in FTIR spectra. Chemometric applications may be a useful solution to partitioning out such simultaneous variation in a quantitative and interpretive manner and has only recently gained traction in the chemistry community for analysis of concurrent reactions[14]. The objective of this study was to investigate the influence of MCC filler loading on the shear strength and thermal properties of MCC reinforced PF composites while the interaction between MCC and the phenolic resin was probed through chemometric analysis.
2.Experimental
2.1. Materials
- The resin used was a commercial Hexion 13BO33 Liquid PF solution intended for bonding wood based composites (Table 1)[4]. MCC was purchased from Sigma-Aldrich Chemicals, Inc., USA. The MCC used was powdery with a particle size in the range of 20-25 µm and its density is 0.4 g/mL. It was stored in sealed containers before use to avoid moisture uptake from the environment.
2.2. Preparation of MCC Reinforced PF Matrix
- All adhesive mixtures (PF and MCC) were prepared by mechanically mixing the aqueous PF with the microcrystalline cellulose at 0, 3, 6 and 10% (wt/wt) loading ratio for 10 min at room temperature. Preliminary mixing found the particulate nature of MCC at loadings greater than 10% to yield a viscosity that would not be practical during industrial application and so 10% was chosen as the upper limit of the experimental design. The adhesive solution was stored in a freezer until it was used in the experiment.
2.3. Neat Adhesive and Composite Characterization
- The solid content of the PF adhesive solution was measured by weighing a known amount of the adhesive into a pre- weighed Petri dish, then heating in an oven at 60 °C for 30 min. After curing, the Petri dish was kept in a desiccator at room temperature for 2 hours and reweighed. The percentage of solids was measured based on the weight after drying relative to the total adhesive weight prior to drying.The density of the adhesive was also determined by measuring a known volume in a graduated cylinder and then measuring the mass of the cylinder with the known volume of adhesive. Density was calculated as the mass of adhesive divided by the volume of adhesive at room temperature.
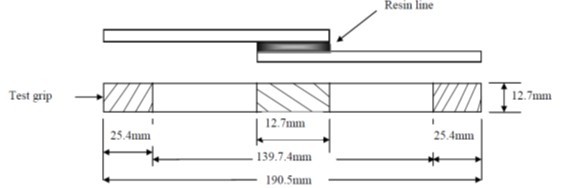 | Figure 1. Diagram of test setup for lap-shear test of adhesive and MCC reinforced adhesives |
2.3.1. Thermal Characterization
- The cure reaction rates of neat adhesive and composite samples were measured using a differential scanning calorimeter (TA Q200, TA instruments, DE, USA). The phenol formaldehyde resin liquid was removed from the freezer and was allowed to warm to room temperature. Liquid samples of 10–15 mg were taken and placed in hermetically sealed aluminum capsules (TA high volume pans) that can withstand vapor pressures up to 10 MPa.Before the test, the temperature of the base heating block was maintained at 40°C. The total area under the exothermal curve, based on the extrapolated baseline at the end of the reaction, was used to calculate the reaction heat at a given temperature. The heat flow (J/g) was measured as a function of curing temperature using a dynamic DSC procedure. The samples were heated to complete cure in a nitrogen environment as defined by the following equation[15]:
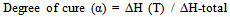 | (1) |
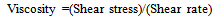 | (2) |
2.3.2. Fourier Transform Infrared Spectroscopy
- Fourier transform infrared spectroscopy (FT-IR) measurements were performed in a Perkin–Elmer Spectrum 400 instrument (Perkin Elmer Co., Waltham, MA) fitted with a single reflectance ATR diamond. The samples (PF and PF/MCC mixtures) were measured immediately after cure to avoid samples picking up moisture from the atmosphere. All measurements were made at a room temperature of 25°C. The contribution of CO2 in air, moisture, and oxygen was eliminated by measuring the background spectra before every test sample. Each spectrum was recorded over 4 scans, in the range from 4000 to 650 cm-1 with a resolution of 4 cm-1. The spectra were then reduced to principal components and regression analysis was performed. Via et al. 2013 can be viewed to better understand the equation development and use of principal components analysis in material assessment[16]. Principal components is method of factor reduction analysis which consolidates the FT-IR spectra into a small number of uncorrelated factors (<10) which can then be used in regression modelling to relate functional groups or analytical values to the performance of the material[17].
2.4. Measurement of Shear Strength
- The adhesive strength of microcrystalline cellulose reinforced resin for the wood-to-wood system was measured by the single lap-shear method and compared with that of untreated phenol formaldehyde resin following ASTM D-1002. Southern yellow pine (Pinusspp) was used in lap fashion to hold the adhesive during the shear test. The dimensions were 1.27 cm wide, 0.162 cm thick and 10.6 cm long (Figure 1) were used in the adhesion study to provide a shear force to the adhesive to cellulose composite. The wood specimens were cut so that the grain was parallel to the length so as to ensure complete failure in the PF - cellulose composite only. The dimension for the overlap area of all samples was 1.27 cm by 2.54 cm. All flakes were conditioned at 65% RH and 22°C.A lap shear specimen was manufactured by bonding two flakes together with MCC reinforced liquid PF adhesive on their smooth surfaces. The amount of adhesive and or adhesive cellulose mixture was 20 ±2 mg and was applied with a metal spatula onto the overlap area at one end of each flake and was controlled to yield a thin, uniform and continuous adhesive film. The two flakes were then lapped over the length of their coated ends.The sample flakes were pressed perpendicularly by a hot press at 150°C and a pressure of 10 N/cm2 for 5 minutes. The samples were then allowed to cool and condition to 65% RH and 22°C. Six test samples were used for each adhesive mixture type as specified in ASTM D-1002 using a Zwick/Roell (Z010) and were tested at 25°C. Only samples with 100% failure in the PF/cellulosic composites were used for analysis.
 | Figure 2. Effect of cellulose loadings on mean viscosity of PF and PF/MCC mixtures with 1 error bar equal to a standard deviation |
3. Results and Discussion
3.1. Effect of Addition of MCC on Viscosity
- The results of the viscosity measurements of the untreated and cellulose loaded PF adhesives are presented in Figure 2. The increase in viscosity with cellulose loading was nearly linear (α=0.05) and gives an indication of the effect of MCC on adhesive-MCC mixture flow properties prior to curing. The addition of 10% MCC to the adhesive matrix nearly quadrupled the viscosity and spraying of the adhesive-MCC mixture would be difficult at loadings at or above 10% and this may be attributable to the increased surface area of the MCC.
3.2. Effect of MCC on Cure Properties
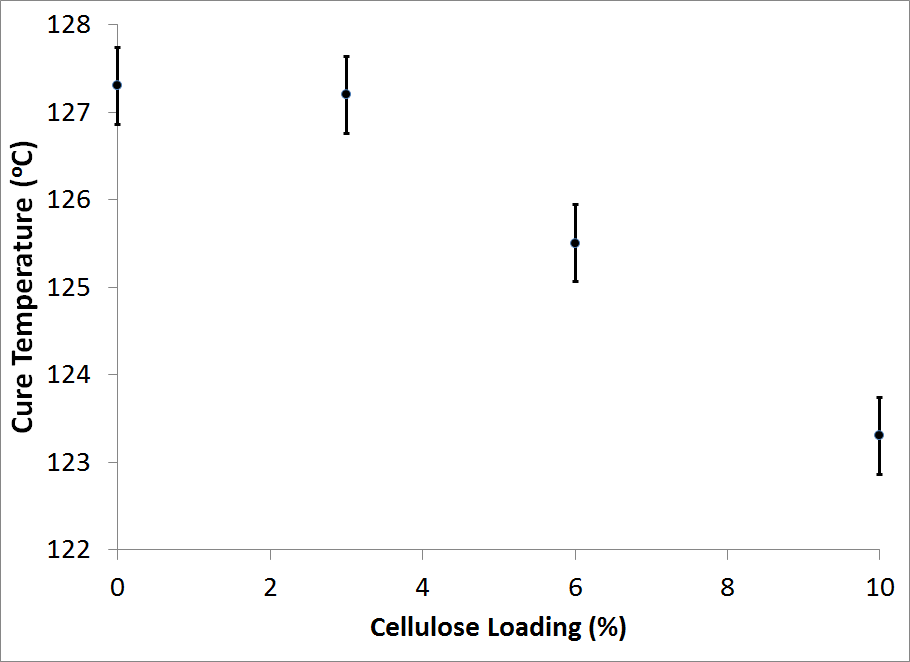
- Figure 3 shows the effect of the cellulose addition on the location of the exothermic maximum peak of reaction (curing temperatures). It can be seen that the curing temperature decreases from 127.3 to 123.7°C when the cellulose loading is increased from 0 to 10 wt%.Likewise, the onset of cure dropped linearly with cellulose addition and was typically 10 to 15°C lower than the endothermic peaks. The area under the peaks of the original DSC graph corresponds to the degree of cure based on the assumption that heat flow (dH/dt) is proportional to the reaction rate (dR/dt) and that polymerization during cure represents the total degree of energy in the thermodynamic system[15].The onset and maximum curing temperatures found in this study was similar to other studies.For instance, Christiansen and Gollob observed two exothermic peaks at 98 to 129°C and 139 to 151°C for liquid phenol formaldehyde adhesives [18]. The authors related the first and primary peak to the methylolation reaction, and the second one to the condensation reaction of the PF adhesive. Figure 4 represents the heat of reaction calculated from the DSC curves for the different cellulose loadings.It can be observed that the addition of 3 wt% cellulose increases the heat of reaction from 904 to 1699 (J/g). While after this increase the heat of reaction decreases for additional cellulose loadings.The maximum in the reaction heat at 3 wt% cellulose loading could be an indication of the optimal ratio of cellulose to PF adhesive and suggests that the addition of cellulose to the biocomcomposite affects the curing process due to some physical or chemical interaction between the cellulose and PF adhesive.
 | Figure 3. Effect of cellulose loadings on cure temperatureof PF and PF/MCC mixtures with 1 error bar equal to a standard deviation |
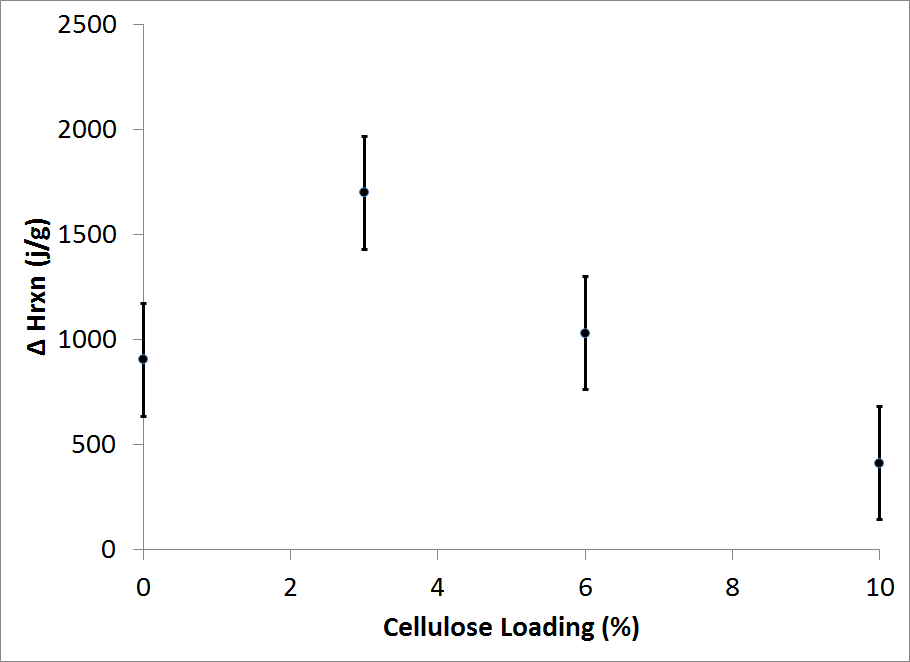 | Figure 4. Heat of reaction calculated from DSC scans at 0, 3, 6, and 10 wt % MCC loading with standard error bars |
3.3. Thermal Degradation of MCC/PF Matrix
- The thermal behavior (i.e. pyrolysis and thermal degradation) of the neat PF and PF reinforced adhesives was measured with TGA at a heating rate of 10°C/min and in nitrogen atmosphere between 30 and 800°C. The thermogravimetric (TG) curves as well as their corresponding 1st derivative thermogravimetric curves (DTG) for the untreated and reinforced PF adhesives are shown in Figure 5a and 4b. A comprehensive evaluation of the TGA thermograms reveals 4 stages of polymer degradation at 150, 230, 430 and 530°C. Analysis of polymer residue after complete heating found the neat PF to exhibit 52% residue while the 3, 6 and 10wt% MCC filled PF had reduced solid residues of 48, 48, and 45% respectively. Despite this slight reduction in degradation resistance, the additional degradation with cellulose was minor and suggests that cellulose addition to PF adhesives may not drastically alter the thermal stability of the polymer in the field.Application of the 1st derivative pretreatment to the thermograms helped to partition 4 stages of thermal degradation if the first peak is discarded (Figure 5b). The first peak in this study occurred between 110 to 140°C and was probably attributable to water evaporation.The following 3 temperatures 230, 430 and 530°C represent the primary peaks associated with PF adhesive degradation. Similarly, another study found three overlapped peaks at 245, 418 and 545°C in the DTG curves for a commercial resol PF adhesive[21].In this study, the peak at 230°C was attributable to the additional cross-links that are formed within the PF matrix. The additional cross-links at this temperature are believed to be the result of condensation reactions between the phenol and methylene group resulting in a carbon-hydrogen crosslink.
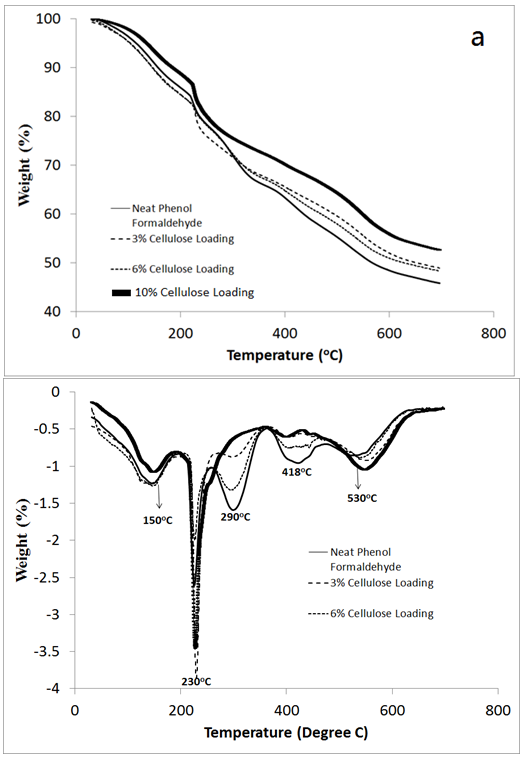 | Figure 5. (a: ) Thermogravimetric (TG) and (b) 1st derivative of TG curves for PF and MCC reinforced PF adhesive |
3.4. Effect of the Addition of MCC on the shear Strength of PF Adhesives
- The shear strength properties of the untreated PF and PF/MCC samples were studied. Figure 6 demonstrates the relationship between increasing cellulose loading and tensile shear. A rapid increase in strength occurred between 3 to 6wt% MCC addition while only a minor increase in strength occurred between 6% and 10% MCC addition. This pattern of shear strength with MCC addition may be at least partially explained by the heat of reaction as measured with DSC (Figure 4).
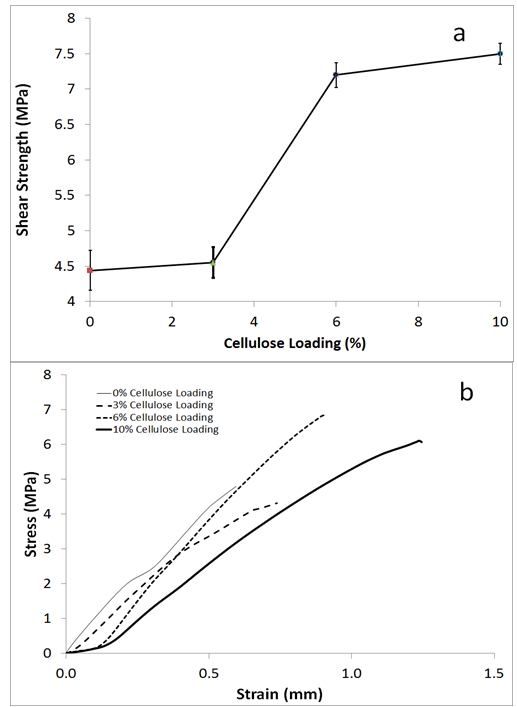 | Figure 6. (a: )Effect of cellulose reinforcement on shear strength of MCC/PF composite and (b) stress strain curves for representative samples |
3.5. FTIR Chemometric Analysis of PF and MCC/PF Composites
- FTIR was used to examine possible interactions between the microcrystalline cellulose and phenol formaldehyde resin. In this experiment, powdered samples of the MCC were used whereas the untreated PF and PF/MCC mixtures were cured before use. Characteristic peaks were assigned based on a literature review[12,13]. Figure 7(a) and (b) shows the bands that were significant (exceeded the dotted line) after the spectra was decomposed into eigenvalues and eigenvectors. The bands observed at 1525 cm-1 were attributable to the C=C aromatic ring that is characteristic of the phenol formaldehyde resin.The bands at 826 cm-1 were attributable to the CH out of plane stretch in the PF resin and this appeared at 815 cm-1 in this study.When PC2 from the FTIR spectra was regressed against cure temperature values (graph not shown) a moderate linear relationship was found (R2 = 0.52; alpha < 0.05) which indicates the significance of the functional groups at 815, 1722, and 2925 cm-1 which is due to the CH and C=O, CH2 stretch within the resin matrix (Figure 7a)[12,13].When PC3 from the FTIR spectra was regressed against the heat of reaction values a moderately strong linear relationship was found (R2 = 0.73; alpha < 0.05) and indicates the importance of functional groups at 1525, 1575, 1722, and 2925 cm-1 on the heat of reaction (Figure 7b).Once again, the bands at 1722 and 2925 cm-1 were important but this time for the heat of reaction and this suggests that the CH2 functional group (2925 cm-1) played a role in connecting cellulose with the PF matrix. This observation is justifiable given that PC3 and PC2 are orthoganol (purely independent) meaning that PC2 accounts for cure temperature variation while PC3 accounts for variation in the heat of reaction. The relationship between heat of reaction and PC3 (with a particular focus on 2925 cm-1) may be explained by He and Riedl who supports that the reaction between the cellulose in the woody cell wall and PF occurs at CH2 and CH functional groups[11]. On the other hand, because significant heat is generated due to methylene bridges that occur within the resin matrix itself, it is difficult to quantify the percentage of variation attributable to crosslinking within the PF resin itself versus that which is due to bonding between MCC and PF[25].But for sure, the nonlinear increase in shear strength supports an improved interaction between cellulose and PF; particularly since the shear strength and reactivity levelled off at 10% MCC loading.Finally, the band at 1525 cm-1 was attributable to the C=C bond within the PF matrix[12].
 | Figure 7. Eigenvector Loadings of (a) Principal Component 2 and (b) Principal component 3.All Principal Components were computed from the unpretreated FTIR spectra at 0, 3, 6, and 10% MCC loading.The dotted line represents ±2 standard deviations from center |
4. Conclusions
- Crystalline cellulose was uniformly dispersed at loadings of 0, 3, 6, and 10% (by weight) in phenol formaldehyde (PF) and tested for thermal and shear strength properties.It was found that the addition of cellulose to untreated PF adhesive increases the viscosity but slightly reduces the cure temperature by 4℃. The heat of reaction was a function of cellulose loading with the maximum heat of reaction occurring at 3% loading. Analysis of the FTIR spectra with principal components analysis found the CH2 functional group (2925 cm-1) to be sensitive to both cure temperature and reactivity (α = 0.05). The thermal stability of the untreated PF adhesive was reduced only slightly with the addition of MCC which may be important for in service/field conditions and applications.Finally, the shear strength of the PF adhesive was increased by a factor of 1.7 with a 10% cellulose loading.
ACKNOWLEDGMENTS
- The authors are thankful for the Robert Lewis Adams Graduate Fellowship, Hatch, McStennis, and AU-IGfunding. The Center for Bioenergy and Bioproducts is also thanked for the use of their laboratory space. Finally, this project was in alignment with the goals of the Forest Products Development Center in which low value biomass can be converted to higher value products.To support these goals, Regions Bank is also acknowledged for their financial assistance.
References
| [1] | Park B.D., Riedl B., Hsu E.W., Shields J., 2001, Application of cure-accelerated phenol-formaldehyde., PF, adhesives for three-layer medium density fiberboard., MDF, manufacture, Wood Science and Technology 35(4):311-323. |
| [2] | Pan H., Shupe T.F., Hse C-Y., 2008, Synthesis and cure kinetics of liquefied wood/phenol/formaldehyde resins, Journal of Applied Polymer Science 108(3):1837-1844. |
| [3] | Pan H., 2011, Synthesis of polymers from organic solvent liquefied biomass: A review, Renewable and Sustainable Energy Reviews 15(7):3454-3463. |
| [4] | Mathew A.P., Oksman K., Sain M., 2005, Mechanical properties of biodegradable composites from poly lactic acid., PLA, and microcrystalline cellulose., MCC, Journal of Applied Polymer Science 97(5):2014-2025. |
| [5] | Marcovich N., Auad M., Bellesi N., Nutt S., Aranguren M., 2006, Cellulose micro/nanocrystals reinforced polyurethane, Journal of materials research 21(04):870-881. |
| [6] | Azizi Samir MAS, Alloin F., Dufresne A., 2005, Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field, Biomacromolecules 6(2):612-626. |
| [7] | Spoljaric S., Genovese A., Shanks R.A., 2009, Polypropylene–microcrystalline cellulose composites with enhanced compatibility and properties, Composites Part A: Applied Science and Manufacturing 40(6):791-799. |
| [8] | Atta-Obeng E., Via B., Fasina O., 2012, Effect of Microcrystalline Cellulose, Species, and Particle Size on Mechanical and Physical Properties of Particleboard, Wood and Fiber Science 44(2):227-235 |
| [9] | Wang S., Xing C., 2010, Wood adhesives containing reinforced additives for structural engineering products, United States Patent #0285295. |
| [10] | Veigel S., Jone R., Weigl M., Gindl-Altmutter W., 2012, Particle board and oriented strand board prepared with nanocellulose-reinforced adhesive, J Nanomaterials 2012:1-8. |
| [11] | He G., Riedl B., 2004, Curing kinetics of phenol formaldehyde resin and wood-resin interactions in the presence of wood substrates, Wood Science and Technology 38(1):69-81. |
| [12] | Holopainen T., Alvila L., Rainio J., Pakkanen T.T., 1998, IR spectroscopy as a quantitative and predictive analysis method of phenol–formaldehyde resol resins, Journal of Applied Polymer Science 69(11):2175-2185. |
| [13] | Poljanšek I., Krajnc M., 2005, Characterization of phenol-formaldehyde prepolymer resins by in line FT-IR spectroscopy, Acta Chimica Slovenica 52(3):238-244. |
| [14] | George G., Hynard N., Cash G., Rintoul L., O’Shea M., 2006, Spectroscopic probes for real-time monitoring of polymer modification and degradation reactions, Comptes Rendus Chimie 9(11–12):1433-1443. |
| [15] | Myers G.E., Christiansen A.W., Geimer R.L., Follensbee R.A., Koutsky J.A., 1991, Phenol–formaldehyde resin curing and bonding in steam-injection pressing. I. Resin synthesis, characterization, and cure behavior, Journal of Applied Polymer Science 43(2):237-250. |
| [16] | Via B.K., Adhikari S, Taylor S., 2013, Modeling for proximate analysis and heating value of torrefied biomass with vibration spectroscopy, Bioresource technology. |
| [17] | Cowe I.A., McNicol J.W., 1985, The use of principal components in the analysis of near-infrared spectra, Applied Spectroscopy 39(2):257-266. |
| [18] | Gollob L., Krahmer R., Wellons J., Christiansen A., 1985, Relationship between chemical characteristics of phenol-formaldehyde resins and adhesive performance, Forest products journal 35(3):42-48. |
| [19] | Allan G.G., Neogi A.N., 1971, The Mechanism of Adhesion of Phenol-Formaldehyde Resins to Cellulosic and Lignocellulosic Substrates, The Journal of Adhesion 3(1):13-18. |
| [20] | Alma M.H., Bastürk M.A., Shiraishi N., 2001, Cocondensation of NaOH-catalyzed liquefied wood wastes, phenol, and formaldehyde for the production of resol-type adhesives, Industrial & engineering chemistry research 40(22):5036-5039. |
| [21] | Chen Y., Chen Z., Xiao S., Liu H., 2008, A novel thermal degradation mechanism of phenol–formaldehyde type resins, Thermochimica Acta 476(1):39-43. |
| [22] | Singh B., Gupta M., 2005, Performance of pultruded jute fibre reinforced phenolic composites as building materials for door frame, Journal of Polymers and the Environment 13(2):127-137. |
| [23] | Carvalho S.S., Dutra J.R., Carvalho A.C., Vieira L.M., Christoforo A.L., 2012, Experimental evaluation of the employment of a laminated composite material with sisal fibers as reinforcement in timber beams, International Journal of Composite Materials 2(5):97-100. |
| [24] | Cheng H., Dowd M.K., Selling G., Biswas A., 2010, Synthesis of cellulose acetate from cotton byproducts, Carbohydrate polymers 80(2):449-452. |
| [25] | Lu X., Pizzi A., 1998, Curing conditions effects on the characteristics of thermosetting adhesives-bonded wood joints—Part 1: Substrate influence on TTT and CHT curing diagrams of wood adhesives, Holz als Roh-und Werkstoff 56(5):339-346. |
| [26] | Liu H., Laborie M-PG., 2011, Bio-based nanocomposites by in situ cure of phenolic prepolymers with cellulose whiskers, Cellulose 18(3):619-630. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML