-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2013; 3(3): 39-45
doi:10.5923/j.cmaterials.20130303.01
Ion-Exchange and Thermal Properties of Electrically Conductive Polyaniline-Titanium(IV) Phosphate Cation Exchange Nanocomposite
Asif Ali Khan, Umair Baig
Analytical and Polymer Research Laboratory, Department of Applied Chemistry, Faculty of Engineering and Technology, Aligarh Muslim University Aligarh, 202002, U.P. India
Correspondence to: Asif Ali Khan, Analytical and Polymer Research Laboratory, Department of Applied Chemistry, Faculty of Engineering and Technology, Aligarh Muslim University Aligarh, 202002, U.P. India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Electrically conductive organic-inorganic polyaniline-titanium (IV) phosphate cation exchange nanocomposite was synthesized by a modified sol-gel technique by incorporating titanium (IV) phosphate precipitate with the matrix of polyaniline. The material showed good electrical conductivity and successfully used in electroanalytical studies. The effect of heating on ion-exchange capacity, effect of eluant concentration, elution behavior, pH titration studies were carried out to understand the ion-exchange capabilities of the exchanger. The degradation process and thermal behavior of polyaniline and polyaniline-titanium(IV)phosphate nanocomposite were also investigated by thermogravimetric analysis (TGA, DTA and DTG). The polyaniline-titanium(IV)phosphate cation exchange nanocomposite showed good ion-exchange behavior and found more thermally stable than pure polyaniline and other materials of this class.
Keywords: Polymer, Nanocomposite, Ion-Exchange Behavior, Thermal Behavior
Cite this paper: Asif Ali Khan, Umair Baig, Ion-Exchange and Thermal Properties of Electrically Conductive Polyaniline-Titanium(IV) Phosphate Cation Exchange Nanocomposite, International Journal of Composite Materials, Vol. 3 No. 3, 2013, pp. 39-45. doi: 10.5923/j.cmaterials.20130303.01.
Article Outline
1. Introduction
- Ion exchange materials are widely used for application in various fields such as fuel cell storage batteries (fuel cells), electrochemical separations, separation of heavy toxic metal ions and in making ion selective membrane electrodes[1-6]. For these applications, thermally and mechanically stable ion exchange materials should be developed. In the recent years, electrically conductive organic-inorganic ion-exchange nanocomposite materials are advanced class of materials due to their extraordinary properties within a single molecular composite[7-18]. Organic polymeric part of the composite provides the mechanical, electrical and chemical stability, where as the inorganic part provides the thermal stability, ion-exchange behaviour and also contributes in the electrical conduction.Polyaniline-titanium(IV) phosphate, a electrically conductive organic-inorganic cation exchange nanocomposite has shown excellent methanol and ammonia sensing properties and successfully used in electroanalytical studies in our previous research work[18-19, 2]. However ion-exchange and thermal properties of this material has yet to be reported in our present research work. The following pages summarize the ion-exchange properties such as effect of eluant concentration, elution behavior and effect of pH and thermal properties such as effect of heating on exchanger and thermogravimetric analysis (TGA, DTA and DTG) of Polyaniline-titanium(IV)phosphate cation exchange nanocomposite. PANI-TiP cation exchange nanocomposite is found more thermally stable than other materials of this class.
2. Experimental
2.1. Reagents and Chemicals
- Aniline (99%) from Qualigens (India Ltd.) was purified by distilling twice before use, titanium oxide (TiO2 from CDH India), ammonium persulphate (C.D.H. A.R. grade) was used as received. All other reagents and chemicals were of analytical grade and were obtained from CDH, Loba Chemie, E-merck or Qualigens (India Ltd.).
2.2. Preparation of Polyaniline-titanium(IV)Phosphate Nanocomposite
- The ‘organic–inorganic’ polyaniline-titanium(IV) phosphate cation exchange nanocomposite was prepared as reported earlier[18-19].
2.2.1. Synthesis of Polyaniline
- The organic polymer polyaniline was prepared by mixing different volumes of the solution of 10% aniline (C6H5NH2) and 0.1M potassium persulphate (K2S2O8) prepared in 1M HCl with continuous stirring by a magnetic stirrer for half an hour at oC, and green colored gels were obtained.The gel was kept for 24 h at 0℃.
2.2.2. Synthesis of Titanium(IV)phosphate
- Preparation of titanium (IV) phosphate (TiP) cation exchanger was carried out by taking different ratios of titanium (IV) sulphate stock solution and 1 M orthophosphoric acid solution prepared in dematerialized water. Titanium(IV)sulphate stock solution was prepared by dissolving 2 g of titaniumdioxide in 62.5 mL of hot concentrated sulfuric acid containing 25 g of ammonium sulphate with constant stirring[20].
2.2.3. Synthesis of Polyaniline-titanium(IV) Phosphate Nanocomposite
- Polyaniline-titanium (IV) phosphate cation-exchange nanocomposite was prepared by the sol-gel mixing of organic polymer polyaniline, into the inorganic precipitate of TiP. In this process, when the gel of polyaniline were added to the white inorganic precipitate of TiP with constant stirring for 1 h, the resultant mixture were kept for 24 hours at room temperature (25+2℃). Now the polyaniline based composite cation-exchanger gels were filtered off washed thoroughly with DMW to remove excess acid and any adhering trace of ammonium persulphate. The washed gels were dried over P4O10 at 45℃ in an oven. The dried products were immersed in DMW to obtain small granules. They were converted to the H+ form by keeping in 1 M HNO3 solution for 24 hours with occasional shaking intermittently replacing the supernatant liquid. The excess acid was removed after several washing with DMW. The materials were finally dried at 40℃ and grinded by pastel mortar to obtain fine powder of composite.
2.3. Ion Exchange Behavior of Polyaniline-titanium(IV) Phosphate Nanocomposite
2.3.1. Effect of Eluant Concentration
- To find out the optimum concentration of the eluant for complete elution of H+ ions, a fixed volume (250 ml) of sodium nitrate (NaNO3) solutions of varying concentration were passed through a column containing 1g of the exchanger in the H+-form with a flow rate of ~ 0.5 ml min-1. The effluent was titrated against a standard alkali solution of 0.1 M NaOH for the H+ ions eluted out (Fig 2).
2.3.2. Elution Behavior
- Since with optimum concentration for a complete elution was observed to be 2 M for sample, a column containing 1g of the exchanger in H+- form was eluted with NaNO3 solution of this concentration in different 10 ml fractions with minimum flow rate as described above and each fraction of 10 ml effluent was titrated against a standard alkali solution for the H+ ions eluted out. This experiment was conducted to find out the minimum volume necessary for almost complete elution of H+ ions, which determines the exchange efficiency of the column as shown in Fig 3.
2.3.3. Effect of pH
- pH titration studies of polyaniline-titanium(IV)phosphate was performed by the method of Topp and Pepper[21]. A total of 500 mg portions of the cation-exchanger in the H+ -form were placed in each of the several 250 ml conical flask, followed by the addition of equimolar solutions of alkali metal chloride and their hydroxides in the different volume ratios, the final volume was kept 50 ml to maintain the ionic strength constant. The pH of the solution was recorded every 24 h until equilibrium was attained which needed ~5 days and pH at equilibrium was plotted against the milliequivalents of OH- ions added (Fig. 3).
2.4. Thermal Behavior of Polyaniline-titanium(IV) phosphate Nanocomposite
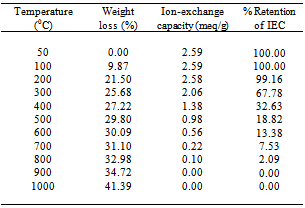
2.4.1. Thermal Effect on Ion-exchange Capacity (IEC)
- To study the effect of temperature on the IEC, 500 mg samples of the cation exchange nanocomposite (PTP-2) in the H+-form were heated at various temperatures in a muffle furnace for 1 h and the Na+ ion-exchange capacity was determined by column process after cooling them at room temperature.
2.4.2. Thermal (TGA, DTA and DTG) Studies
- The degradation process and thermal behavior of polyaniline and polyaniline-titanium(IV)phosphate nanocomposite were investigated by the thermogravimetric analysis (TGA), differential thermal analysis (DTA) and differential thermal gravimetry (DTG) using thermal analyzer-V2.2A (Du Pont 9900), heating sample from ~24℃ to ~1000℃ at the rate of 10℃/min in the nitrogen atmosphere at the flow rate of 200 ml/min.
3. Results and Discussion
- To find out the optimum concentration of the eluant for complete elution of H+ ions, a fixed volume (250 ml) of sodium nitrate (NaNO3) solution of varying concentrations were passed through a column containing 1 g of the exchanger in the H+-form with a flow rate of ~ 0.5 ml min−1. The effluent was titrated against a standard alkali solution of 0.1M NaOH for the H+ ions eluted out. A maximum elution was observed with the concentration of 2.0 M NaNO3 as indicated in Fig. 1.Since with optimum concentration for a complete elution was observed to be 2.0 M for sample PTP-3, a column containing 1 g of the exchanger in H+-form was eluted with NaNO3 solution of this concentration in different 10 ml fractions with minimum flow rate as described above and each fractions of 10 ml effluent was titrated against a standard alkali solution for the H+ ions eluted out. This experiment was conducted to find out the minimum volume necessary for almost complete elution of H+ ions, which determines the exchange efficiency of the column as shown in it is clear from Fig. 2 that maximum volume to release complete hydrogen ion of 1g ion exchanger was found 130 ml NaNo3 The pH titration curves for PANI-TiP (PTP-3) were obtained under equilibrium conditions with NaOH/NaCl, KOH/KCl and LiOH/LiCl, systems indicated bifunctional behavior of the material as shown in Fig. 3. For the sample PTP-3, the rate of H+–Na+ exchange was faster than those of H+–K+ and H+–Li+ exchangers.In order to study the effect of heating on the ion-exchange capacity, the dried PANI-TiP cation exchange nanocomposite was heated at rising temperature, in between the ion-exchange capacity was calculated at different temperatures. The detail of weight loss and ion-exchange capacity is given in Table 1, where it can be seen that the nanocomposite showed steady weight loss, which started from 100℃ and till 1000℃ almost ~41.39% degradation took place. The initial weight loss may be due to the loss of water vapor and volatile impurities and after which degradation of polymer chains may have taken place. It can be interpreted that the cation exchange nanocomposite is fairly stable upto 300℃ as it retained ~67.78% of its ion-exchange capacity and ~74.32% of its initial mass.Fig. 4 shows the TGA curves of PANI and PANI-TiP nanocomposite. In the case of PANI, there are two stages of weight loss, the first weight loss till 300oC, probably due to physisorbed water molecules evaporated at this temperature. The second weight loss up to 800℃ can be ascribed to the degradation of the polymers unsaturated groups whereas beyond 800oC PANI is stable up to 980℃. The PANI-TiP nanocomposite was initially stable upto 400℃ (2.5% weight loss); thereafter, they decompose gradually at 850℃ because of degradation of PANI and subsequently remain stable up to 980℃. The total mass loss up to 980℃ has been estimated to be about 38.40% and 60.20% for the PANI and PANI-TiP nanocomposite. As results, these data confirm that the presence of TiP in the PANI-TiP nanocomposite is responsible for the higher thermal stability of the composite material in comparison with pristine PANI.Fig. 5 shows the DTA curve of pure PANI and PANI-TiP nanocomposite. DTA of PANI was found to exhibit one endothermic peak at 83℃ (-1.58 μV) and one exothermic peak at 249℃ (8.78 μV). The endothermic peak at 83℃corresponds to decomposition stage of PANI between 24℃ to 1000℃ as shown in TGA while the exothermic peak at 249℃corresponds to decomposition stage of PANI from 200℃ to 300℃ as indicated in TGA (Fig. 4). However, PANI-TiP nanocomposite exhibited one endothermic peak 70℃ (-2.2 μV) corresponds to decomposition stage between (22℃ t0 100℃) and two exothermic peaks at 124℃ (0.90 μV) and 738℃ (19.53 μV) corresponds to decomposition stage between (100℃ t0 200℃) and (700℃ to 800℃) respectively as shown in TGA (Fig. 4).DTG analysis of pure PANI and PANI-TiP nanocomposite was studied as a function of rate of weight loss (μg/min) versus temperature (Fig. 6). In case of pure PANI decomposition at 76℃, 262℃ and 528℃ was found with 274.6μg/min, 161.6μg/min and 124.0μg/min weight loss respectively. However, in the case of PANI-TiP nanocomposite, the decomposition was observed at 65℃, 117℃, 743℃ and 969℃ with 147.6μg/min, 152.1μg/min, 14.9μg/min and 119.4μg/min weight loss respectively. Thus, it could be concluded from the DTG studies that the rate of thermal decomposition was higher in the case of PANI-TiP nanocomposite, whereas in the case of pure PANI, the rate of thermal decomposition is lower. Better thermal resistance of pure PANI-TiP nanocomposite was due to incorporation of TiP in the PANI matrix.
|
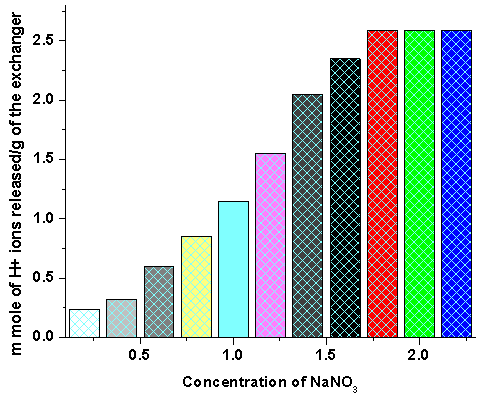 | Figure 1. Effect of eluant concentration on the ion-exchange capacity of PANI-TiP cation exchange nanocomposite |
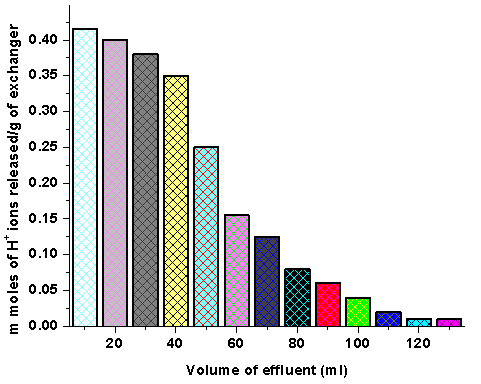 | Figure 2. The elution behavior of PANI-TiP cation exchange nanocomposite |
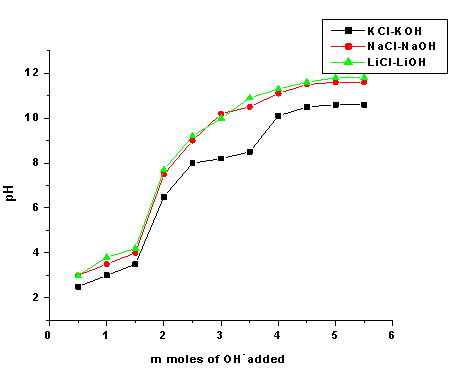 | Figure 3. pH-titration curves for PANI-TiP cation exchange nanocomposite with various alkali metal hydroxides |
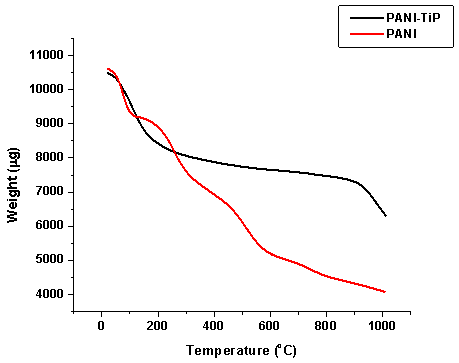 | Figure 4. TGA of PANI and PANI-TiP cation exchange nanocomposite |
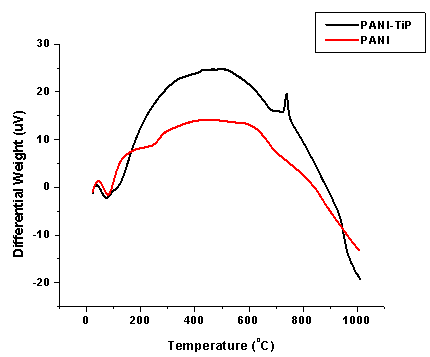 | Figure 5. DTA of PANI and PANI-TiP cation exchange nanocomposite |
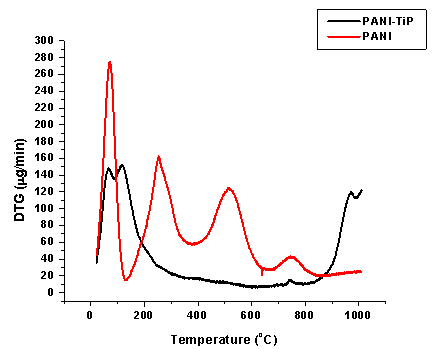 | Figure 6. DTG of PANI and PANI-TiP cation exchange nanocomposite |
4. Conclusions
- In the present paper, we have presented a detailed study of ion-exchange and thermal properties of electrically conductive PANI-TiP cation exchange nanocomposite. The results of effect of eluant concentration, effect of pH and analysis of elution behavior were showed good ion-exchange capabilities of the PANI-TiP nanocomposite. The addition of TiP also changes the thermal properties of PANI-TiP cation exchange nanocomposite. The PANI-TiP cation exchange nanocomposite found more thermally stable than pure polyaniline and other materials of this class.
ACKNOWLEDGEMENTS
- This work was supported by Ministry of Environment and Forest (19-36/2007-RE) and University Grants Commission. The authors are thankful to department of applied chemistry, Z.H.College of Engg. &Technology, A.M.U. (Aligarh) for providing research facilities.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML