-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2012; 2(6): 137-141
doi: 10.5923/j.cmaterials.20120206.04
Study of Influence of Copolymer Vinyl Chloride and Vinyl Acetate on the Properties of Butadiene Nitrile Rubber
Shiraz M. Mammadov1, Sehrana A. Rzayeva1, Adil A. Garibov1, Oktay N. Akperov2, Tunzala F. Gojayeva2, Jovdat S. Mammadov1, Nushaba M. Hajiyeva1
1Institute of Radiation Problems of ANAS, Baku, AZ1143, Azerbaijan
2Baku State University, Baku, AZ 1143, Azerbaijan
Correspondence to: Shiraz M. Mammadov, Institute of Radiation Problems of ANAS, Baku, AZ1143, Azerbaijan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
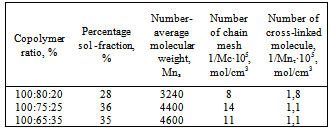
Influence of a copolymer of vinyl chloride and vinyl acetate to the properties of butadiene nitrile rubber has been studied. It is shown that the growth of the molecular weight of BNR by mixing latex rubber and copolymer in a ratio 80:20, 75:25, 65:35 leads to obtaining micro heterogeneous systems. Copolymer initially acts as an interstructural amplifier and then depending on their ratio plays the role of the dispersed phase in the plasticization. By the method of rheological analysis it was found out that increase of molecular weight was observed in the interval time (0-90 min), characteristic viscosity increases with the ratio of 75:25 copolymer from 0.6 to 1.4. A sol-gel analysis showed that with increase in the concentration of the copolymer in BNR the number of chains grid (1/Ms) in the rubber decreases. It was found out that consumption of cross linked molecules (1/Mn) for the cross-linking is reduced, it becomes constant at a ratio of 100:65:25. Comparison of the kinetics of thermometallic oxide vulcanization of BNR with the accelerator disulphide chloride benzene (DSCB) increases the yield concentration of the effective cross-links (n `c) in the molecule BNR. Experiment showed that in the presence of the accelerator DSCB and zinc oxide the structuring mainly goes by the double bond.
Keywords: Butadiene Nitrile Rubber, Vinyl Chloride, Vinyl Acetate, Viscosity, Rheology, Vulcanization, Spectroscopy
Cite this paper: Shiraz M. Mammadov, Sehrana A. Rzayeva, Adil A. Garibov, Oktay N. Akperov, Tunzala F. Gojayeva, Jovdat S. Mammadov, Nushaba M. Hajiyeva, "Study of Influence of Copolymer Vinyl Chloride and Vinyl Acetate on the Properties of Butadiene Nitrile Rubber", International Journal of Composite Materials, Vol. 2 No. 6, 2012, pp. 137-141. doi: 10.5923/j.cmaterials.20120206.04.
1. Introduction
- It is known[1-10], that the vinyl compounds containing active and polar groups interact strongly with the hydrocarbon molecular part of polymer. The structuring of elastomers is observed in the presence of compounds containing two or more active halogen atom and acetate, thus it is possible to obtain sufficiently strong elastomeric materials having a high resistance to the heat ageing, abrasion and dynamic endurance[11-17]. The study of occurring at the same chemical reaction makes it possible to facilitate significantly the development of cross-linking system for manufacturing materials with predetermined properties. In the literature[1, 11-22] the large number of various substances are described which promotes acceleration of the chemical reaction in butadiene nitrile elastomers, for example, with polyvinyl chloride (VC). With filling polyvinyl chloride butadiene nitrile rubber (BNR) enhances ozone resistance of elastomer and helps to create a solid structure and to form ozone protective film on its surface. In the literature[1, 11-22] the large number of various substances are described which promotes acceleration of the chemical reaction in butadiene nitrile elastomers, for example, with polyvinyl chloride (VC). With filling polyvinyl chloride butadiene nitrile rubber (BNR) enhances ozone resistance of elastomer and helps to create a solid structure and to form ozone protective film on its surface. The systematic researches of the effect of unsaturated elastomers filled with copolymer vinyl chloride with vinyl acetate (VA) to the output parameters of the grid, the changes of the molecular structure, rheological properties and also the content of the double bonds in the polyblend (mixture of elastomer with vinyl chloride and vinyl acetate) was not carried out. It is presented to study the effect of interaction of copolymer of vinyl chloride with vinyl acetate on the elastic, structural parameters of the grid and rheological properties of butadiene nitrile elastomer.
2. Experimental
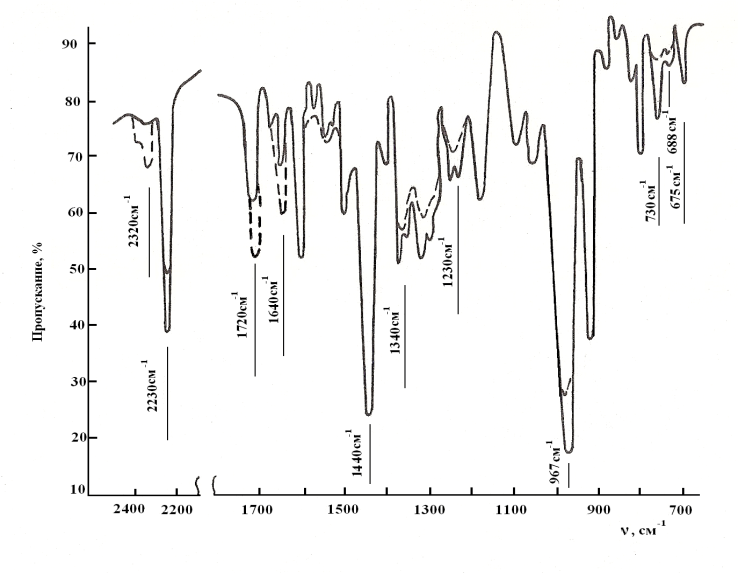
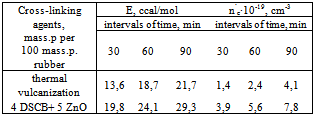
- The butadiene nitrile rubber filled with copolymer of vinyl chloride with the vinyl acetate is used as an object of research. Filled BNR is obtained during joint coagulation of butadiene nitrile latex (with the content of the bound acrylonitrile 37-40 %) with latex of polyvinylchloride (PVC) and poly vinyl acetate (PVA) in the ratio of 80:20, 75:25, 65:35. According to the research results carried our by IK method -spectroscopy in a butadiene part of polymer upon polymerization of the isometric composition of double bonds in the filled system was: 1,2-isomer of 16 %, 1,4-isomer of 18,7 % and trance 1,4 isomer. Macromolecules of SKN-40 rubber consists of statically allocated parts of butadiene, acrylonitrile and filled with vinyl chloride copolymer with the vinyl acetate. Features after the structuring BNR influence to their microstructure, molecular weight, molecular mass distribution (MMR) and gel content. Therefore, before processing researches in filled BNR, number-average and weight-average molecular weight and polydispersity (Мn-69 thousand; Мw-226 thousand; Мw/Мn=4.1) has been defined by gel permeation chromatography (GPC) beforehand.BNR filled with PVC copolymer and (PVA) is expanded in the ratio 75:25, after 15-20 minutes of plasticization the obtained mixture is produced compression of the sample at 143К in the range 0-90 minutes during 20 minutes. For studying kinetic of structuring the 3 mass. part system of disulphonic acid chloride benzene (DSHB) + 5 mass. part ZnO on 100 mass. part of rubber has been used. The characteristic viscosity of BNR filled with copolymer was determined in toluene at 293К by known procedures on Ubbelohde type of viscosimeter. The calculation was carried out on Marx Hauvink's equation[η] = kMα at value of the constant K=4,9∙104 and α = 0,64 for toluene[23]. Studying elastic formation properties were carried out according to GOST 10722-74. Entrance of concentration of cross-linkage (n'c); number of the cross-linked molecules (1/Мnτ) was determined by a sol-gel analysis. The calculation of parameters of a spatial grid of cross-linked elastomer was determined by Flory-Raineri formula[24]. The change of the molecular structure, filled BNR was determined by IR method of infrared spectroscopy (IRS) in the range of 700-3000cm-1. For this filled BNR was dissolved in toluene during 24hrs. The rubber film was obtained by applying a solution onto a substrate and a constant evaporation of solvent. The boxes of KBr were used as a substrate during the measurement of spectrum in the range of 600-2000cm and the boxes of LiF and NaCl in the range of 2000-3000cm. The substrate with a film was fixed the holder and placed in the sample compartment in the spectrophotometer. Identification of the spectra was carried out in accordance with correlation tables[25-27].
3. Results and Discussion
- Put forward[7] the colloidal-chemical concept of anomalous growth of viscosity BNR during filling of copolymer vinyl chloride with the vinyl acetate in works is reduced the fact to the following: Fillers (copolymers) are limited compatible with the polymers in the field of the concentration, corresponding to molecular solubility. Copolymers (vinyl chloride and vinyl acetate) of the correct reinforcement of rubber increase free volume of polymer, and more, the dense packing of its molecular structure. The increase of a ratio of concentration of copolymer in a polymeric matrix leads to the formation of micro heterogeneous system. Copolymer (filler) first plays a role of the inter structural amplifier, and then the role of fulfilling the disperse phase at plasticization depending on their ratio.Based on this situation and granting various ratios of filler (copolymer) in BNR, it is necessary to expect:a) Extremum existence on curve viscosity - a filler ratio only in limited area of temperature test;b) Dependence of effect of structural changes on transverse strain τ; c) Dependence of viscosity of the filled rubber on time and temperature of storage of a sample.Carried-out experimental researches in properties of the butadiene nitrile rubbers (BNR-40) filled with copolymer vinyl chloride and vinyl acetate, confirmed the mentioned assumptions. On figure 1, 2, 3 shown the dependence of elastic characteristics of BNR, filled with copolymer vinyl chloride and vinyl acetate. One can see that the copolymer chosen in various ratios of copolymer causes change of properties of BNR in plasticity (figure 1), hardness (figure 2) and Mooney viscosity (figure 3). Comparatively observable small growth of plasticity (figure 1) and a decrease of hardness (figure 2) at copolymer ratios 80:20 and 75:25 in BNR lead to change these characteristics on regularities, usual for BNR. According to[1] for mechanical solubility (plastification) to rise in the rate of the gel above 70% leads to the destruction of chains, which leads to the destruction of the chains that partly different effects on molecular weight and mechanical - mechanical processes during the dissolution of the copolymers.
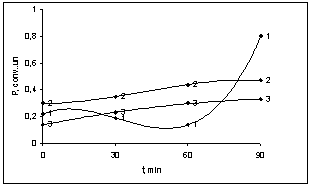 | Figure 1. The dependence of plasticity of BNR filled with various ratios of the copolymer of vinyl chloride and vinyl acetate by heating during 90 min; 1- 80:20, 2- 75:25, 3- 65:35 |
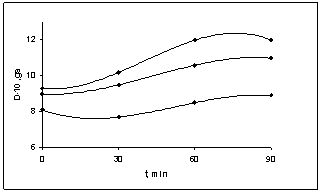 | Figure 2. The dependence of hardness of BNR filled with various ratios of the copolymer of vinyl chloride and vinyl acetate by heating during 90 min; 1- 80:20, 2- 75:25, 3- 65:35 |
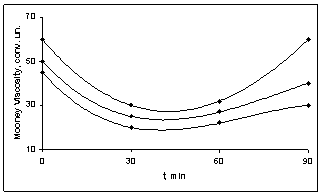 | Figure 3. The dependence of viscosity of BNR filled with various ratios of the copolymer of vinyl chloride and vinyl acetate by heating during 90 min; 1- 80:20, 2- 75:25, 3- 65:35 |
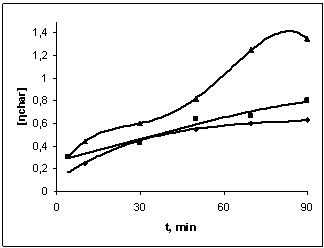 | Figure 4. Dependence of the characteristic viscosity of BNR filled with copolymers of vinyl chloride and vinyl acetate after heating at 423K during 90 min; 1- 65:35, 2- 75:25, 3- 80:20 |
|
|
4. Conclusions
- The present work shows that by mixing of latex BNR and filled vinyl chloride and vinyl acetate in a ratio of 80:20, 75:25, 65:35 leads to formation microheterogeneous systems, acting as a dispersed phase in mechanical dissolution (plastification).It is shown that the shear stress of solubility vinyl chloride copolymers and vinyl acetate in the rubber, observed the rise in the rate of the gel (70%) of the elastomer system, which leads to the destruction of the chains of BNR. Decreasing of the molecular weight of the polymer mass at a shear stress on the rollers influence differently to the rheological properties such as plasticity (figure 1), hardness (figure 2) and of Mooney viscosity (figure 3). With duration of the structuring in mode 423K∙90`, in a filled elastomer system observed an increase in molecular weight. Characteristic viscosity is increased in samples 2 (75:25) from 0.6 to 1.4. With increasing time, the molecular weight of the samples filled copolymers at 70 min is constant, but then, with increasing time of the process of structuring observed decreasing of molecular weight in all samples. Filled copolymers of vinyl chloride and vinyl acetate with BNR mainly determined by polar groups-C ≡ N, C-Cl and their reactivity at mechanical, chemical and thermal influences.Comparison kinetics of thermometal oxide vulcanization of BNR with participation of amplifier disulphide chloride benzyl (DSCB) show the increasing of cross links yield (n`c) in the molecule of BNR. By method of sol-gel analysis determined that in the presence of reinforcing DSCB and zinc oxide are primarily structured by a double bond and the formation of new active centres are suppressed by metal oxides or the products of their transformation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML