-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2012; 2(6): 127-136
doi:10.5923/j.cmaterials.20120206.03
Rheological Properties of Hybrid Hydrogels of Weakly Charged Polyelectrolytes Crosslinked by Aluminoxane Particles
S. S. Radchenko, I. A. Novakov, Ph. S. Radchenko,
Department of Analytical and Physical Chemistry and Physical Chemistry of Polymers, Volgograd State Technical University, 400005, Russian Federation
Correspondence to: S. S. Radchenko, Department of Analytical and Physical Chemistry and Physical Chemistry of Polymers, Volgograd State Technical University, 400005, Russian Federation.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The rheological behavior of hybrid hydrogels of weakly charged polyelectrolytes with aluminoxane particles (APs) formed by noncovalent interactions has been studied. This interaction observed for the polyelectrolyte Praestol-2500 is the most pronounced and is due to strong electrostatic noncovalent bonds between positively charged APs and anionic groups of the copolymer. By the methods of capillary, rotational, and vibrational rheometry, it has been ascertained that two processes compete: the compacting of coils of macromolecules and their crosslinking through APs. The latter process dominates at relatively high concentrations of reagents, and depends on ratios between polymer units and APs and the presence of a low-molecular-mass indifferent electrolyte (NaCl). Formed hybrid hydrogels relate to physical gels with the unstable nature of the network of physical bonds, and under the action of shear stress demonstrate “Weissenberg effect.” The method of dynamic mechanical analysis with the use of a micro-Fourier rheometer has shown that the ratio storage modulus – loss modulus depends on the amount of AP sol and its ratio to the quantity of copolymer in the initial mixture. A scheme for spatial structure hybrid hydrogels has been suggested.
Keywords: Hybrid Hydrogels, Weakly Charged Polyelectrolytes, Aluminoxane Particles
Cite this paper: S. S. Radchenko, I. A. Novakov, Ph. S. Radchenko,, Rheological Properties of Hybrid Hydrogels of Weakly Charged Polyelectrolytes Crosslinked by Aluminoxane Particles, International Journal of Composite Materials, Vol. 2 No. 6, 2012, pp. 127-136. doi: 10.5923/j.cmaterials.20120206.03.
Article Outline
1. Introduction
- At present, the creation of composite materials is characterized by the use of nanodisperse materials of both inorganic and organic nature. Such composites are often referred to as hybrid nanomaterials.[1] This term is often related to purely inorganic nanocrystals and semiconductors, [2],[3] organic nanocomposites,[4],[5] and mixed disperse systems. In the latter case, it is assumed that the composite includes metal or metal oxide (hydroxide) particles as the disperse phase, whereas a polymer or its solution plays the role of the continuous phase.[6-10]Polymeric organo–inorganic composites belong to the promising class of hybrid materials due to a unique combination of magnetic, catalytic, nonlinear optical, and sensoric properties of inorganic nanoparticles with a set of properties of the polymeric matrix and its ability to stabilize inorganic clusters dispersed in it.There are a lot of methods for obtaining nano-objects and their composites.[11-14] The interaction of linear organic macromolecules with aqueous dispersions of charged nanoparticles (hydrosols) may be regarded as a possible approach to the production of organo–inorganic nanocomposites.This method looks attractive because (i) it makes possible the controllable arrangement of nanoparticles in a polymeric matrix, that is, via its reaction centers and (ii) the so-called polymer–colloid complexes (PCCs) (for brevity sake, named polycomplexes) that are formed in this case are generated spontaneously due to noncovalent interactions.[15-17] The formation of polycomplexes may proceed during the sol–gel synthesis of nanoparticles in the presence of polymers[18-20] or through the simplest method—via the blending of a sol with a polymer solution.[15],[21-23] Exactly this method was used to prepare polycomplexes of some types of water-soluble polymers with aluminoxane particles (APs) in the sols of superbase polyaluminum hydroxychloride (PAHC).[24-26] These complexes represent the colloidal dispersion of APs formed during the hydrolysis of some salts of aluminum as a result of the polycondensation of aluminum aqua hydroxo complexes and are distinguished by high aggregate stability at a wide range of concentrations and temperatures.[27] On the basis of small-angle X-ray scattering studies, APs may be related to physical fractals having an internal self-similar structure with a fractal dimensionality df = 1.0 and a radius of gyration rg = 1.6 nm.[28] Various kinds of noncovalent interactions are typical for APs. As a consequence, they may form polycomplexes both with nonionogenic polyacrylamide (PAA)[24],[26] and with its cation-active[29] and anion-active[30] copolymers. However, soluble polycomplexes are obtained only at definite ratios of APs to functional groups of copolymers. Otherwise, insoluble polycomplexes are formed if unlikely charged reagents are used or polycomplexes are not formed at all because of large forces of repulsion of likely charged reagents. Polycomplexes of PAHC sols with polyacrylamide show promise for practice as regulators of the stability of disperse systems, for example, in the processes of purification of natural water and sewages,[31-33] in enhanced oil recovery techniques[34],[35] and as environmentally friendly binding agents for disperse materials.[36] In all the above-listed cases, hybrid polycomplexes are used in the form of aqueous suspensions or gels, that is, fluid systems. At the same time, no data are available on the rheological behavior of these new systems. For most real liquids, irreversible deformations, which appear during flow, are characterized by the complicated dependence of different factors, and their rheological behavior is often in the intermediate region between the liquid and solid. This feature is especially pronounced for high-molecular-mass polymers, aqueous solutions of which possess viscoelastic properties. The pattern of the flow of polymer solutions often determines the possibility of their processing and application. Therefore, the rheological study of such systems is of great importance for practice. Moreover, in addition to its main direct purpose—determination of the rheological characteristic of fluid systems—rheology may serve as a structural method that relates rheological parameters and structural changes in a deformed system.[37] The goal of this work was to study the rheological behavior of aqueous solutions and hydrogels of polymer–colloid complexes of APs with weakly charged polyelectrolytes. As far as particles in a polycomplex occur in the chemically bound via noncovalent bonds state rather than in the inert form, they naturally affect the conformational state of polymer macromolecules. Therefore, to compare the rheological properties of a polymer and its polycomplex, aqueous solutions of initial polymers were also investigated. As it is known, for polymer solutions, the regions of diluted and semidiluted solutions exist outside the crossover region, where the elastic properties of polymer liquids begin to manifest themselves. Therefore, capillary and rotational viscometry and the method of dynamic mechanical analysis with the use of a micro-Fourier rheometer were applied as investigation procedures.
2. Experimental
2.1. Initial Materials
- A dispersion of APs was prepared from an aluminum alloy in the form of an aqueous sol containing 13.5 wt% Al3+ and with the atomic ratio Cl-/Al3+= 0.45. The technique of preparation and the characteristics of the sol as well as the dimensional and fractal characteristics of APs were described in[27],[28].As weakly charged polyelectrolytes, commercial samples with characteristics listed in Table 1 were employed; they were used without any additional purification. An aqueous solution of polyacrylamide was prepared via the free-radical polymerization of acrylamide (99.9%; Alfa Aesar, USA) in an aqueous solution (3 wt% acrylamide) in the presence of potassium persulfate (97%; Alfa Aesar, USA) at a temperature of 60°C and was used without additional purification. Sodium chloride of special purity grade (99.99%; Alfa Aesar, USA) was used. In all experiments, twice-distilled water was used. Polycomplexes were prepared by mixing aqueous solutions of polyelectrolytes of different concentrations with preset quantities of the AP sol. After shaking in a shaker, aqueous dispersions (gels) were allowed to stand at room temperature for a day.
2.2. Rheological Investigations
- Viscometric measurements were performed by means of a Brookfield DV-II+Pro (USA) rotary viscometer equipped with a UL (Ver. Ro4 s/s) adapter and a thermostated cell. Experimental data were processed with the use of a DVLOADER authorized program. The yield stress was determined on a YR-1 special-purpose rheometer equipped with a set of V-71-73 vane spindles. The measurement data were processed by means of a YR-1-4AY authorized program. Viscoelastic properties were investigated on an MFR-2000 micro-Fourier rheometer (GBS, Australia). Measurements were performed in the frequency interval 0–100 Hz with a step of 1 Hz at a temperature of 25°C. All variable parameters and calculations of measurable characteristics were enclosed in an MFR-2100 Ver. 1.1b4 authorized program.
3. Results and Discussion
- Polymer–colloid complexes of APs with water-soluble polymers are thermodynamically stable compounds with a variable composition in the case of soluble polycomplexes[26] or with a characteristic composition when polycomplexes are insoluble.[38] As was shown previously, polycomplexes may be soluble either if nonionogenic polyelectrolytes[25] or weakly charged polyelectrolytes[30] are used. In both cases, polycomplexes are transparent homogeneous disperse systems with specific physicochemical features peculiar to every component. One of them is the aggregative stability of each of the dispersion phases. The specific feature of the given system is that the sol of PAHC is a known regulator of the stability of dispersion systems, whereas a water-soluble polymer is often used as a flocculant in the separation of dispersion systems. The rheological behavior of each of the reagents may change in the presence of other reagents. Moreover, both reagents are polyelectrolytes; hence, they are influenced by the ionic strength of the solution. The superposition of such specific features on the properties of interacting reagents may cause inadequate dependences. Therefore, both interacting disperse systems—AP sols and aqueous solutions of polyelectrolytes—as well as polycomplexes, the products of their interaction, were investigated separately.
3.1. Investigation of Rheological Characteristics of the Sol of Aluminoxane Particles
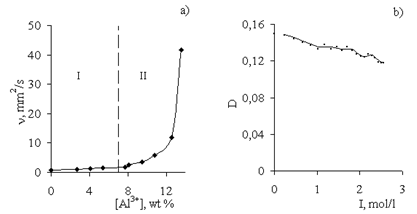
- Figure 1 presents the experimental data on the kinematic viscosity of the AP dispersion measured at various concentrations of the disperse phase (Al3+), and demonstrates the effect of a low-molecular-mass indifferent electrolyte (NaCl) on the aggregative stability of the sol.
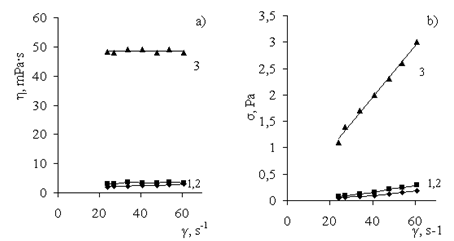 | Figure 2. Curves for (a) viscosity and (b) flow of AP sol at different concentrations of disperse phase (Al3+, wt%): (1) 4.5, (2) 6.8, and (3) 13.5. T = 30°C |
 | Figure 3. Curves for viscosity (η) and flow (σ) of PAHC gel ([Al3+] = 13.5 wt%) upon the addition of NaCl solution with concentrations (wt%): (1) 0, (2) 0.9, (3) 1.8, (4) 2.7, (5) 3.6, and (6) 4.5 |
3.2. Rheological Properties of Aqueous Solutions of Weakly Charged Polyelectrolytes and Their Complexes with Aluminoxane Particles
- The behavior of polyelectrolytes in aqueous solutions is determined by the conformational state of macromolecules and their concentrations. In this study, we selected three kinds of water-soluble polymers: nonionogenic polyacrylamide, weakly anionic Praestol-2500, and weakly cationic Praestol-611 BC. Some of their characteristics are given in[29],[34]. It should be noted that the characteristics of commercial samples are tentative. Thus, Praestol-2500 relates to nonionogenic polymers. However, as follows from potentiometric analysis, 1.2 mol% COOH groups are contained in Praestol-2500. Hence, this compound may be related to weakly anionic polymers that form polycomplexes similarly to weakly charged copolymers of acrylamide with acrylic acid.[30] This is also the reason why polyacrylamide prepared under laboratory conditions via the free radical polymerization of reactive acrylamide was used as a nonionogenic polymer. In this case, its molecular mass was significantly smaller than those of commercial Praestol-2500 and Praestol-611 BC. As evidenced by potentiometric analysis, this polymer was free of COOH groups. Soluble polymer–colloid complexes of APs with water-soluble polymers are thermodynamically stable compounds with variable compositions. Their behavior in aqueous solutions should evidently differ from the behavior of corresponding linear polymers. In the case of strongly diluted solutions, the intramolecular interaction of macromolecules with APs accompanied by the compaction of macromolecules may occur. As is known, under thermodynamically favorable conditions, with an increase in the polymer concentration, a network of topological entanglements appears between separate chains of macromolecules.[41] The concentration of crossover C* is a characteristic value of this state (Table 1).
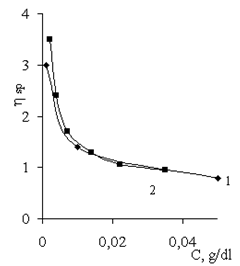 | Figure 4. Dependence of specific viscosity of Praestol-611 BC solution on the nature and concentration of electrolytes: (1) NaCl and (2) PAHC. CPraestol-611 BC = 0.05 g/dl |
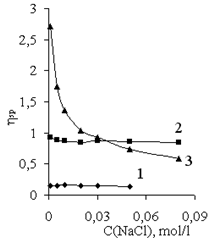 | Figure 5. Dependence of specific viscosity of diluted solutions of polymers (0.05 g/dl) on the concentration of NaCl in solution (mol/l): (1) polyacrylamide, (2) Praestol-2500, and (3) Praestol-611 BC |
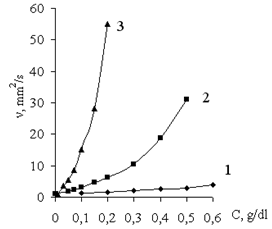 | Figure 6. Kinematic viscosity (v) of aqueous solutions of polycomplexes versus concentration of polymers (C): (1) PAA, (2) Praestol-2500, and (3) Praestol-611 BC |
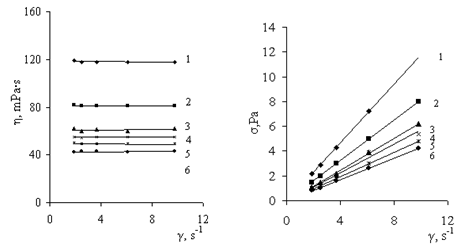
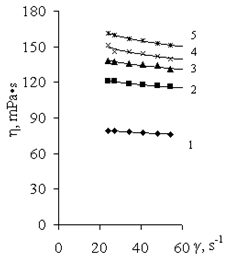 | Figure 7. Curves for viscosity of solutions of (1) PAA (2 g/dl) and its PCCs at various ratios (PAA unit: Al3+ (mol/mol)): (2) 1:0.5, (3) 1:1, (4) 1:2, and (5) 1:4 |
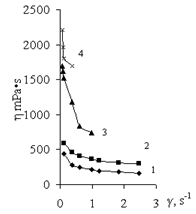 | Figure 8. Curves for viscosity of solutions (1) of Pr-2500 (0.6 g/dl) and its polycomplexes with AP at various ratios (Pr-2500 unit: Al3+ (mol/mol)): (2) 1:0.1, (3) 1:0.25, and (4) 1:0.37 |
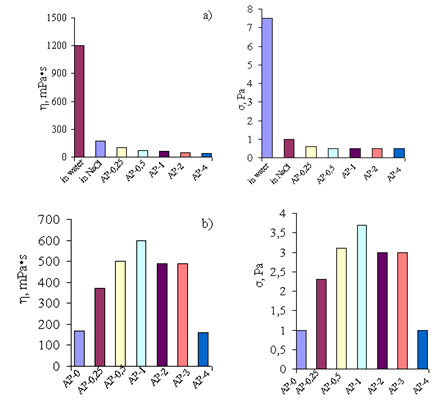 | Figure 9. Dynamic viscosity (η) and shear stress (σ) in (a) aqueous and (b) water–salt solutions of Pr-611 (1% NaCl) upon addition of the AP sol. The concentration of Pr-611 BC is 0.9 g/dl. Designations AP-O–AP-4 correspond to the ratio[Pr611 BC:Al3+] in moles |
 | Figure 10. The picture of the Weissenberg effect arising under the action of shear stress in the gels of polyelectrolyte crosslinked by aluminoxane particles |
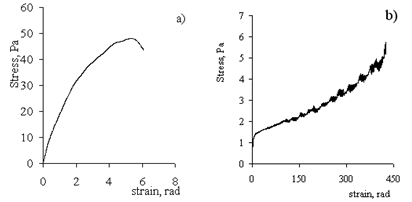 | Figure 11. Dependence of shear stress on the deformation of gel based on (a) Pr-2500 and (b) Pr-611. The concentration of copolymers in water–salt solution is 1.2 g/dl. The ratio of copolymer unit:Al3+ = 2:1 (mol/mol). The shear rates are 1.5 rpm and 3.0 rpm for Pr-2500 and Pr-611, respectively |
|
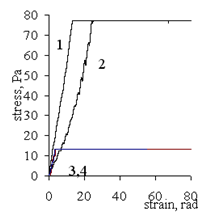 | Figure 12. Dependence of shear stress on the strain of gel based on Pr-2500 at different ratios Pr-2500 unit:Al3+ (mol/mol): (1) 1:0.1, (2) 1:0.5, (3) 1:1, and (4) 4:1. The shear rate is 1.5 rpm |
 between the shear stress and strain for viscoelastic liquids should vary from
between the shear stress and strain for viscoelastic liquids should vary from 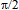 (the viscous medium) to 0 (the elastic medium). For the gels under consideration, the viscoelastic deformation is evidently determined by two parameters: polymer concentration and ratio between crosslinking agents (AP) and polymer.
(the viscous medium) to 0 (the elastic medium). For the gels under consideration, the viscoelastic deformation is evidently determined by two parameters: polymer concentration and ratio between crosslinking agents (AP) and polymer. 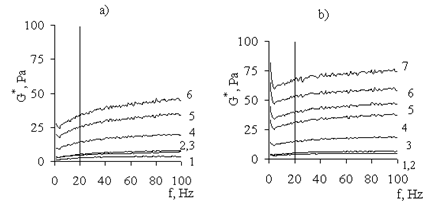 | Figure 13. Dependence of complex modulus (G*) on frequency (f) for water–salt solutions of (a) Pr-2500 and (b) its PCC at polymer concentrations of (1) 0.3, (2) 0.6, (3) 0.9, (4) 1.2, (5)1.5, (6) 1.8, and (7) 2.0 |
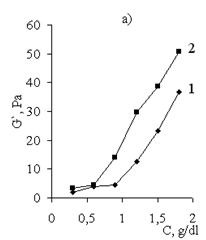 | Figure 14. Storage modulus (G`) vs. concentration of (1) Pr-2500 and (2) its polycomplex |
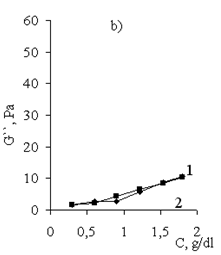 | Figure 15. Loss modulus (G``) vs. concentration of (1) Pr-2500 and (2) its polycomplex |
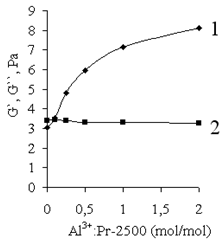 | Figure 16. Storage modulus (G`) and loss modulus (G``) vs. AP (Al3+):Pr-2500 (mol/mol) in the initial mixture. The concentration of Pr-2500 is 0.9 g/dl |
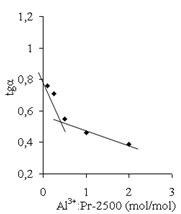 | Figure 17. The value of tg(α) vs. AP (Al3+):Pr-2500 unit molar ratio. The polymer concentration is 0.9 g/dl |
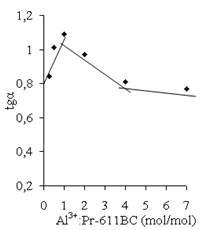 | Figure 18. The value of tg(α) vs. AP (Al3+):Pr-611 BC unit molar ratio. The polymer concentration is 0.9 g/dl |
4. Conclusions
- Aluminoxane nanosized particles form stable bonds with polymer chains due to noncovalent interactions that lead to the formation of an extended three-dimensional network rather than form another stable phase in a mixture with aqueous solutions of polyelectrolytes. Aluminoxane nanosized particles in the sol PAHC carry out a part of polyelectrolytes in the initial mixture with acrylamide copolymers and cause the compacting of coils of macromolecules. At that time, noncovalent interactions of APs with units of macromolecules result in their crosslinking. A domination that or another process depends on the type of ionic groups and the presence of a low-molecular-mass indifferent electrolyte (NaCl). As a result, “soft” nanocomposite materials appear in the form of optically transparent gels, in which organic and inorganic components interact at the molecular level. Such organo–inorganic hydrogels lack their own shape, and their yield stress is determined by the nature of the three-dimensional network that appears through the interactions of positively charged aluminoxane clusters with the functional groups of polymers. Structurization takes place under the conditions of a single-phase state of the disperse system stabilized by stable hydrogen bonds in an aqueous solution. Formed hybrid hydrogels possess viscoelastic characteristics and under the action of shear stress show the “Weissenberg effect.” The ratio storage modulus – loss modulus depends on the amount of the AP sol and its ratio to the quantity of the copolymer in the initial mixture. Inorganic aluminoxane particles under consideration are structurally similar to silica particles formed during the sol–gel synthesis from tetramethoxysilane; however, they differ in the sign of the surface charge, which is negative for silica particles and positive for aluminoxane particles.
 | Figure 19. Scattering particles in sol: a) silica (rd = 4–6 nm, df = 2.5)[47]; b) aluminoxane particles (rd = 2.2 nm and df = 1.0)[28] |
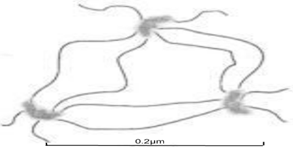 In such a scheme, aluminoxane particles play the role of network junctions that bind polyelectrolyte macromolecules into a common spatial network. It is clear that the density of such a network is determined by the amount of particles, and its strength depends on the nature of noncovalent bonds of particles with polymer chain units, which are the most pronounced for column interactions typical for weakly anionic Pr-2500.
In such a scheme, aluminoxane particles play the role of network junctions that bind polyelectrolyte macromolecules into a common spatial network. It is clear that the density of such a network is determined by the amount of particles, and its strength depends on the nature of noncovalent bonds of particles with polymer chain units, which are the most pronounced for column interactions typical for weakly anionic Pr-2500.
References
| [1] | Richard M. Laine, C. Jeffrey Brinker, Emmanuel P. Giannelis (eds.) “Organic/Inorganic Hybrid Materials”, Material Research Society, Warrendale, 2000. |
| [2] | Krishna K. Haldar, Tapasi Sen, Amitava Patra, “Metal Conjugated Semiconductor Hybrid Nanoparticle-Based Fluorescence Resonance Energy Transfer”, J. Phys. Chem. C, vol.114, pp.4869-487, 2010. |
| [3] | Vladimir V. Terekhin, Olga V. Dementeva, Viktor M. Rudoy, “Formation Of Ordered Nanoparticle Assemblies By Block Copolymer Lithography Methods”, Russ. Chem. Rev., vol.80, pp. 453-472, 2011. |
| [4] | Arthi Jayaraman, Kenneth S. Schweizer “Effective Interactions and Self-Assembly of Hybrid Polymer Grafted Nanoparticles in a Homopolymer Matrix”, Macromolecules, vol.42, pp. 8423-8434, 2009. |
| [5] | Claudiu B. Bucur, Zhijie Sui, Joseph B. Schlenoff, “Ideal Mixing in Polyelectrolyte Complexes and Multilayers: Entropy Driven Assembly”, Amer. Chem. Soc., vol.128, pp. 13690-13691, 2006. |
| [6] | Camila Alves de Rezende, Lay-Theng Lee, Fernando Galembeck, “Silica Nanoparticles at Interfaces Modulated by Amphiphilic Polymer and Surfactant”, Langmuir, vol.24, pp. 7346-7353, 2008. |
| [7] | Bruno Alonso, Franck Fayon, Dominique Massiot (eds.) “Hybrid Organic−Inorganic Mesostructured Membranes: Interfaces and Organization at Different Length Scales”, J. Phys. Chem. C, vol.114, pp. 11730-11740, 2010. |
| [8] | Takami Akagi, Kazuki Watanabe, Hyungjin Kim, Mitsuru Akashi, “Stabilization of Polyion Complex Nanoparticles Composed of Poly(amino acid) Using Hydrophobic Interactions”, Langmuir, vol.26, pp. 2406-2413, 2010. |
| [9] | Patrick J. Colver, Catheline A. L. Colard, Stefan A. F. Bon, “Multilayered Nanocomposite Polymer Colloids Using Emulsion Polymerization Stabilized by Solid Particles”, J. Amer. Chem. Soc., vol.130, pp. 16850-16851, 2008. |
| [10] | Yuriy Román-Leshkov, Manuel Moliner, Mark E. Davis, “Hybrid Organic−Inorganic Solids That Show Shape Selectivity”, Chem. Mater., vol.22, pp. 2646-2652, 2010. |
| [11] | Bindushree Radhakrishnan, Rajesh Ranjan, William J. Brittain, “Surface Initiated Polymerizations From Silica Nanoparticles” Soft Mater., vol.2, pp. 386-396, 2006. |
| [12] | Sergey S. Ivanсhev, Saul Ya. Khaikin (eds.), “Preparation Of Nanocomposites By Alkoxysilane Hydrolysis In A Polypropylene Matrix” Polymer. Sci. Ser.A., vol.44, pp. 996-1001, 2002. |
| [13] | Anatolii D. Pomogailo, Aleksandr S. Rosenberg, Gul’zhian I. Dzhazdinmaieva, “Thermolysis Of Metallopolymers And Their Precursors As A Method For The Preparation Of Nanocomposites” Russ. Chem. Rev., vol.80, pp. 257-291, 2011. |
| [14] | Vladimir V. Vinogradof, AleksandrV. Agafonov, Aleksandr V. Vinogradov, “Sol-Gel Synthesis Of Nanostructured Materials Based On Aluminum Oxide With Preset Texture Properties”, Protection of Metals and Physical Chemistry of Surfaces, vol.46, pp. 582-586, 2010. |
| [15] | John C. Heckel, Lydia M. Kisley, Joseph M. Mannion, George Chumanov, “Synthesis and Self-Assembly of Polymer and Polymer-Coated Ag Nanoparticles by the Reprecipitation of Binary Mixtures of Polymers”, Langmuir, vol.25, pp. 9671-9676, 2009. |
| [16] | Marián Sedlák, Čestmír Koňák, “A New Approach to Polymer Self-assembly into Stable Nanoparticles: Poly(ethylacrylic acid) Homopolymers”, Macromolecules, vol.2, pp. 7430–7438, 2009. |
| [17] | Immanuel Willerich, Franziska Gröhn, “Molecular Structure Encodes Nanoscale Assemblies: Understanding Driving Forces in Electrostatic Self-Assembly”, J. Am. Chem. Soc., vol.133, pp. 20341–20356, 2011. |
| [18] | Ivan M. Papisov, Klaudia.I. Bolyachevskaya, Andrey.A. Litmanovich (eds.), “Structural Effects In Matrix Polycondensation Of Silisic Acid”, Eur. Polym. J., vol.35, pp. 2087-2094,. 1999. |
| [19] | Hideharu Mori, Axel H. E. Müller, Joachim E. Klee, “Intelligent Colloidal Hybrids via Reversible pH-Induced Complexation of Polyelectrolyte and Silica Nanoparticles”, J. Am. Chem. Soc., vol.125, pp. 3712-3713, 2003. |
| [20] | Yang-Yen Yu, Wen-Chang Chen, “Transparent Organic–Inorganic Hybrid Thin Films Prepared From Acrylic Polymer And Aqueous Monodispersed Colloidal Silica”, Mater. Chem. and Phys., vol.82, pp. 388–395, 2003. |
| [21] | Ivan M. Papisov, Andrey A. Litmanovich, “On Recognition Phenomena In Polymer–Minute Particle Interactions And Pseudo-Matrix Processes”, Coll. and Surf., vol.151, pp. 399-408, 1999. |
| [22] | Jean-Francois Berret, “Stoichiometry of Electrostatic Complexes Determined by Light Scattering”, Macromolecules, vol.40, pp. 4260-4266, 2007. |
| [23] | Elmar Poselt, Steffen Fischer, Stephan Foerster, Horst Weller, “Highly Stable Biocompatible Inorganic Nanoparticles by Self-Assembly of Triblock-Copolymer Ligands”, Langmuir, vol.25, pp. 13906-13913, 2009. |
| [24] | Ivan A. Novakov, Philipp S. Radchenko, Ivan M. Papisov, “Formation of Polycomplexes Based Polyacrylamide and Aluminum Salts”, Polymer Science Ser. A, vol.45, pp. 805-808, 2003. |
| [25] | Ivan A. Novakov, Philip S. Radchenko, Andrey S. Pastukhov, Ivan M. Papisov, “The Properties Of Aqueous Solutions Of Polymer-Colloid Complexes Of Polyacrylamide With Poly (Aluminum Hydroxychloride)”, Polymer Science, Ser. A, vol.47. pp. 57-60, 2005. |
| [26] | Ivan A. Novakov, Philipp S. Radchenko, Ivan M. Papisov, “A Study Of The Composition OfPolyacrylamide-Polyaluminum Chloride Polymer-Colloid Complexes”, Polymer Science, Ser. B,. vol.49. pp. 111-113, 2007 |
| [27] | Svetlana O. Zakharchenko, Evgeniay A. Litmanovich, Philipp S. Radchenko, (eds.), “Photon Correlation Spectroscopic Study Of The Aggregative Stability Of Colloidal Particles Of Aluminum Pentahydroxide Chloride”, Colloid Journal, vol.68, pp. 425-429, 2006. |
| [28] | Aleksandr S. Ozerin, Philipp S Radchenko, Galina I. Timofeeva, Ivan A.Novakov, “A Study Of Structural And Molecular Weight Characteristics Of Poly(Aluminum Hydroxychloride) Nanoparticles By Small-Angle X-Ray Scattering And Sedimentation Analysis”, Nanotechnologies in Russia, vol.4.pp. 93-101, 2009. |
| [29] | Stanislav S. Radchenko, Ivan A. Novakov, Philipp S. Radchenko, Le Van Cong, (eds.), “Interaction of aluminoxane particles with weakly charged cationic polyelectrolyte”, J. Appl. Polym. Sci., vol.121, pp. 475-482, 2011. |
| [30] | Ivan A. Novakov, Philipp S. Radchenko, Aleksandr S. Ozerin, ElenaV. Rybakova, Stanislav S Radchenko, “Formation Of Polymer–Colloid Complexes Of Aluminoxane Particles With Poly(Acrylic Acid) And Its Copolymers With Acrylamide”, Coll. Polym. Sci., vol.289, pp. 1197-1203, 2011. |
| [31] | Ivan A. Novakov, Stanislav S. Radchenko, Philipp S. Radchenko, “Water-Soluble Polymer-Colloid Complexes Of Aluminum Polyhydroxochloride And Polyacrylamide In Separation Of Model And Real Dispersions”, Russian Journal of Applied Chemistry, vol.77, pp. 1699-1705, 2004. |
| [32] | Stanislav S. Radchenko, Ivan A. Novakov, Philipp S. Radchenko, Elena V.Rybakova, Patent 2288182, RF, 2006. |
| [33] | Stanislav S. Radchenko, Ivan A. Novakov, Philipp S. Radchenko, Le Van Cong, Elena V. Rybakova, “Flocculating Properties of Water-Soluble Polymer-Colloid Complexes of Aluminoxane Particles with Weakly Charged Cationic Polyelectrolytes”, J. of Water Res. and Prot., vol.3, pp. 213-221, 2011. |
| [34] | Stanislav S. Radchenko, Ivan A. Novakov, Philipp S. Radchenko, Aleksandr S. Ozerin, Pavel S. Zeltser, Sergey U. Jakubovski, Patent 2348792, RF, 2009. |
| [35] | Ivan A. Novakov, Stanislav S. Radchenko, Philipp S. Radchenko, Aleksandr S. Ozerin, Aleksey B. Karaulov, “Polyacrylamide-Aluminum Pentahydroxochloride-Urea Formulations As Waterproofing Agents For Oil Pool”, Russian Journal of Applied Chemistry, vol.81, pp. 1389-1394, 2008. |
| [36] | Nikolay A. Kidalov Philipp S. Radchenko Viktor F. Zakutaev, Ivan A. Shamrey Stanislav S. Radchenko Patent 2449854 RF, 2012. |
| [37] | Gebhard Schramm, “A Practical Approach to Rheology and Rhometry”, Gebrueder HAAKE GmbH, Karlsrue, 1994. |
| [38] | Ivan A. Novakov, Philipp S. Radchenko, Aleksandr S. Ozerin, Elena V. Rybakova, “Interaction Of Aluminum Polyhydroxochloride Sol And Poly(4-Vinylbenzene Sulfonic Acid) Sodium Salt”, Polymer Sci. A., V.53, pp. 364-368, 2011. |
| [39] | Nikolay U. Bikadorov, Stanislav S. Radchenko, Ivan A. Novakov, Philipp S. Radchenko, Ol’ga K. Gohova, Patent 2210539, RF, 2005. |
| [40] | Stanislav S. Radchenko, Nikolay U. Bikadorov, Ivan A. Novakov, Ol’ga K. Gohova, (eds.), “Structuring In Concentrated Solutions Of High-Basicity Aluminum Hydroxychloride And In New Coagulant Formulations On Its Base”, Russian Journal of Applied Chemistry, vol.75. pp. 529-534, 2002. |
| [41] | Michael Rubinstein., Ralph H. Colby, “Polymer Physics”, Oxford, N.-Y., Oxford Univer. Press, 2003. |
| [42] | Aleksandr Y. Grosberg, Aleksey R. Khokhlov, “Statistical Physics of Macromolecules”, N-Y, AJP Press, 1994. |
| [43] | Michael J. Gidley, “Molecular Mechanisms Underlying Amylose Aggregation And Gelation”, Macromolecules, vol.22, pp. 351-358, 1989. |
| [44] | Aleksey S. Makarov, Irina A. Andreeva, Viktor Yu. Tretinnik, “Rheological Properties of Polymer-Containing Aqueous Aerosil Dispersions”, Colloid Jornal, vol.63, pp. 731-737, 2001. |
| [45] | Nikolai V. Churaev, “Surface Forces And Physicochemistry Of Surface Phenomena”, Russ. Chem. Rev., vol.73, pp. 25-42, 2004. |
| [46] | Klaas Te Nijenhuis, H. Henning Winter, “Mechanical Properties At The Gel Point Of A Crystallizing Poly(Vinyl Chloride) Solution”, Macromolecules, vol.22. pp. 411-414, 1989. |
| [47] | Irina V. Bakeeva, Ludmila A. Ozerina, Aleksandr N. Ozerin, Vitalii P. Zubov, “Structure And Characteristics Of Organic-Inorganic Hybrid Hydrogels Based On Poly(N-Vinylcaprolactam)-SiO2”, Polymer Science Ser. A, vol.52, pp. 496-505, 2010. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML