-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Composite Materials
p-ISSN: 2166-479X e-ISSN: 2166-4919
2012; 2(3): 32-36
doi: 10.5923/j.cmaterials.20120203.03
Transport Properties of Conductive Polyaniline Nanocomposites Based on Carbon Nanotubes
S. B. Kondawar, M. D. Deshpande, S. P. Agrawal
Department of Physics, RTM Nagpur University, Nagpur - 440033, India
Correspondence to: S. B. Kondawar, Department of Physics, RTM Nagpur University, Nagpur - 440033, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Intrinsically conducting polymers have been studied extensively due to their intriguing electronic and redox properties and numerous potential applications. To improve and extend their functions, the fabrication of multifunctional conducting polymer nanocomposites has attracted a great deal of attention with the advent of nanoscale dimension. In this paper we report the comparative study of nanocomposite synthesized by an in-situ oxidative polymerization of aniline monomer in the presence of functionalized multiwall carbon nanotubes (MWCNT) with that of pure polyaniline (PANI). Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and x-ray diffraction (XRD) are employed to characterize the pure PANI and the PANI-CNT nanocomposite. XRD and SEM reveal the homogeneous coating of PANI onto the CNT indicating that carbon nanotubes were well dispersed in polymer matrix. The interaction between the quinoid ring of PANI and the MWCNT causes PANI chains to be adsorbed at the surface of MWCNT, thus forming a tubular core surrounding the MWCNT was confirmed from FTIR. Nanocomposite shows high electrical conductivity compared to pure PANI. The enhancement in conductivity of the nanocomposite is due to the charge transfer effect from the quinoid rings of the PANI to the MWCNT. The effect of MWCNT on the transport properties of PANI in the form of the transport parameters such as charge localization length, most probable hopping distance and charge hopping energy in the temperature range 300-430 K was also studied.
Keywords: Carbon Nanotubes, Conducting Polymer, Polyaniline, Nanocomposites
Article Outline
1. Introduction
- Organic polymers capable of conducting electricity due to partial oxidation or reduction (i.e., doping), are prospective classes of advanced materials. They possess an extended π-conjugation along the polymer backbone and exhibit semiconducting behaviour. During the last few decades, electrical transport in such conducting polymers has been thoroughly studied[1–4]. Organic conducting polymers exhibit the electrical and optical properties of the metals and retain the attractive mechanical properties of polymers leading to wide range of technological applications. After the report of preparation of carbon nanotubes and polymer composites by Ajayan et al[5], there have been efforts to combine carbon nanotubes and polymers to produce functional composite materials with desirable electrical and mechanical properties[6-8]. Hence some properties of polymer have been exploited by incorporating the nanomaterials into the polymer matrix. Carbon nanotubes have attracted considerable attention due to their potential application in electronic devices. They have unique structural,mechanical, electronic, and thermal properties and are attractive building blocks for the development of novel polymer-nanocomposite materials with enhanced functionality, especially if it comes to enhanced conductivity, thermal stability, and reinforcement properties[9-12]. Sambhu Badra et al[13] reported the superiority of polyaniline over all other conducting polymers. Polyaniline (PANI) is taken as matrix material for our work, because not only it is highly stable in air and in some solvents, but also exhibits dramatic changes in its electronic structure and physical properties Polymerization in the presence of carbon nanotubes leads to a more planar conformation of PANI along multiwall carbon nanotubes (MWCNT). Because of the formation of conducting polymer-CNT networks, these materials are of interest for electronic applications including photovoltaic cells, organic light emitting diodes, electromagnetic shielding, electro static dissipation, antennas, and batteries[14-16]. There is a lot of work reported on the preparation of polyaniline-CNT composites to improve the electrical conductivity of the host polymer, but no one reported the change in transport parameters of the composites. Yi Zhou et al[17] reported the strong interaction in conjugated systems greatly improves the charge-transfer reaction between polyaniline and the carbon nanotube prepared by an in-situ polymerization of aniline monomers usingmulti-walled carbon nanotubes with minimized defects as templates. Polyaniline coated multiwalled carbon nanotubes as additives has been used for enhancing the electrical conductivity of nylone 6 composite was studied by R. R. Vankayala et al[18]. Qi Jianga et al reported the preparation of carbon nanotube/polyaniline nanofiber by electrospinning[19]. Modification of glassy carbon electrode with polyaniline/multi-walled carbon nanotubes composite prepared by Liang Ding et al[20]. Subhodh Srivastva et al[21] reported multiwall carbon nanotube (MWNT) doped polyaniline (PANI) composite thin films for hydrogen gas sensing applications. Due to van-der Waals forces, tight bonding of CNT limits their applications. In most of the work reported, MWCNTs have not functionalized before incorporated into PANI matrix. Therefore in our study MWCNT surface is functionally modified by ultrasonication using H2SO4 and HNO3 to provide specificity for improved interaction between CNT and polymer matrix which enhances the processability and properties of composites. This work describes the synthesis and characterization of protonic acid doped PANI functionalized MWCNT fabricated by an in-situ chemical oxidative polymerization method. First time we present the detailed electrical transport properties[22] of PANI/MWCNT composites, in the form of the transport parameters such as dc electrical conductivity (σ), charge localization length (α-1), most probable hopping distance ( R ) and charge hopping energy (w) in the temperature range 300-430 K.
2. Experimental
2.1. Materials and Methods
- Aniline (99%) and ammonium persulphate (APS) (99%) were purchased from Merck. Aniline was distilled before use for polymerization. High purity MWCNT of diameter 30-40 nm was made available from NPL, New Delhi, India. Other supplement chemicals were of AR grade and used as received.X-ray diffraction (XRD) analysis was carried out on Philips PW1710 automatic X-ray diffractometer with Cu-Kα radiation (λ = 1.5404 Å), with a scanning speed of 10 min−1. FTIR spectra were performed on Shimatzu FTIR-8101A Spectrophotometer in the wavelength range of 400–4000 cm−1. SEM images were taken on JEOL JSM-6360 analytical scanning electron microscope. The electrical conductivity of the compressed pellets of PANI-MWCNT nanocomposites was determined by four probe resistivity technique. The pellets were prepared with the help of hydraulic press (Kimaya Engineers, India) by applying a pressure of 5000 kg/cm2.
2.2. Functionalization of MWCNT
- The solution of 6M H2SO4 and 6M HNO3 in 3:1 ratio was stirred for 10 minute. MWCNT was added to it and then solution was sonicated for 4 hours at 50oC. After centrifugation MWCNT was filtered, washed and dried to get functionalized MWCNT.
2.3. Synthesis of PANI-MWCNT Nanocomposite
- The synthesis of PANI-MWCNT nanocomposite was performed using in-situ oxidative polymerisation by dispersing 100 mg of functionalized MWCNT in 1 M HCl in order to have a monomer/MWCNT weight ratio of 100/1, and using a monomer/oxidant molar ratio of 1/1 as described by Zein et al[23]. The pure conducting polymer (PANI) was synthesised by following the same steps carried out for the nanocomposite without MWCNT. The final products obtained from the syntheses were the emeraldine salt forms of PANI-MWCNT nanocomposite and PANI pure polymer.
3. Results and Discussion
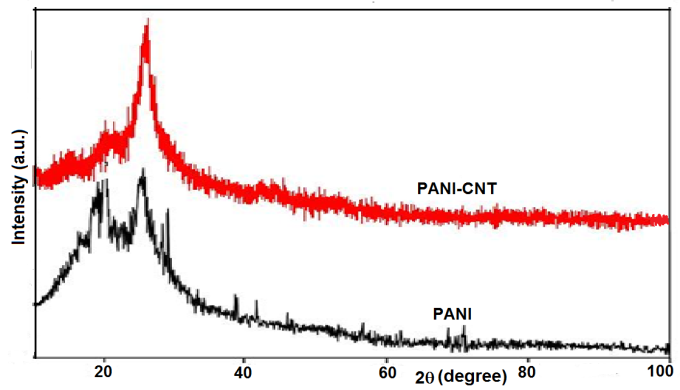 | Figure 1. XRD pattern of PANI-CNT and pure PANI |
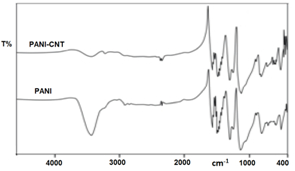 | Figure 2. FTIR spectra of PANI-CNT and pure PANI |
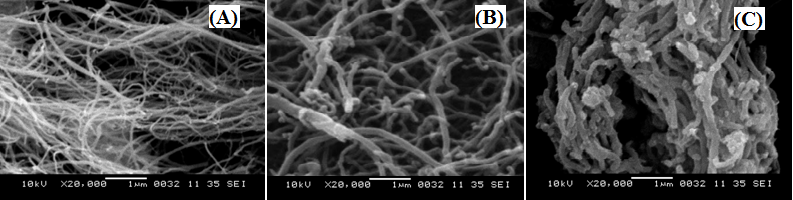 | Figure 3. SEM of MWCNT (A), functionalized MWCNT (B), and PANI-CNT composite (C) |
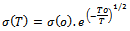 The plot of log σ(T) versus T-1/2 was found to be linear for pure PANI and PANI-CNT nanocomposite, hence To was determined from the slope of the line. ‘To’ the characteristic temperature, can be used to calculate the transport parameters such as charge localization length ( α-1 ), most probable hopping distance ( R ) and charge hopping energy (w) using the following relations,
The plot of log σ(T) versus T-1/2 was found to be linear for pure PANI and PANI-CNT nanocomposite, hence To was determined from the slope of the line. ‘To’ the characteristic temperature, can be used to calculate the transport parameters such as charge localization length ( α-1 ), most probable hopping distance ( R ) and charge hopping energy (w) using the following relations,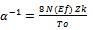
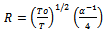
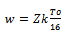 In these relations, Z is the number of nearest neighbouring chains (~4), k is Boltzmann constant and N(Ef) is the density of states per electron volt (2–ring unit suggested for polyaniline)[25]. MWCNT may serve “conducting bridge” connecting the PANI conducting domain. Thus functionalized MWCNT embedded in the PANI matrix have better conductivity with enhanced solubility and processability as compared to that of pure PANI.
In these relations, Z is the number of nearest neighbouring chains (~4), k is Boltzmann constant and N(Ef) is the density of states per electron volt (2–ring unit suggested for polyaniline)[25]. MWCNT may serve “conducting bridge” connecting the PANI conducting domain. Thus functionalized MWCNT embedded in the PANI matrix have better conductivity with enhanced solubility and processability as compared to that of pure PANI.
|
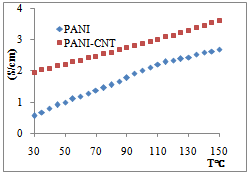 | Figure 4. Electrical conductivity (σ) of PANI and PANI-CNT composite |
4. Conclusions
- PANI-CNT nanocomposite was successfully synthesized by an in-situ chemical oxidative polymerization of aniline. FTIR, XRD and SEM show the effective structural modification and confirm the coating of PANI layer on the CNT surface. Incorporation of functionalized CNT into PANI matrix improved the transport properties of the nanocomposite. Transport parameters of PANI-CNT nanocomposite shows the composite is better electronic material than pure polyaniline.
Acknowledgements
- The authors gratefully acknowledge UGC, New Delhi (India) for financial assistance provided to carry out this work through major research project file F No.39-540/2010 (SR).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML