| [1] | Yip, E., and Cacioli, P., 2002, The Manufacture of Gloves from Natural Rubber Latex., J Allergy Clin. Immunol., 110 (2), S3-S14 |
| [2] | Popović, R., Milenković, D., Popović, R., Plavšić, M., 2005, Properties of Natural Rubber/Carboxilated Styrene-Butadiene Lattices Blends., Scientific-Technical Review, LV (3-4), 66-69 |
| [3] | Jayanthy, T. and Sankaranarayanan, P.E., 2005, Measurement of Dry Rubber Content in Latex Using Microwave Technique., Measurement Science Review, 5 (3), 50-54 |
| [4] | Khalid, K.B., and Daud, W. M., 1994, Dielectric Properties of Natural Rubber Latex at Frequencies from 200MHz to 2500 MHz., Journal of Rubber Research Institute of Malaysia 7 (4), 281-289 |
| [5] | Selvin, T.P., Kuruvilla, J., Sabu, T., 2004, Mechanical Properties of Titanium Dioxide-Filled Polystyrene Microcomposites., Materials Letters 58, 281-289 |
| [6] | Erdem, B., Hunisicker, R. A., Simmons, G.W., Sudo,l E.D., Dimonie, V.L., El-Aasser, M.S., 2001, XPS and FTIR Surface Characterization of Tio2 Particles Used in Polymer Encapsulation., Langmuir, 17, 2664-2669 |
| [7] | Laachachi, A., Cochez, M., Ferriol, M., Lopez-Cuesta, J. M., Leroy, E., 2005, Influence of TiO2 and Fe2O3 Fillers on the Thermal Properties of Poly (Methyl Methacrylate) (PMMA)., Materials Letters, 59, 36-39 |
| [8] | Džunuzović, E., Jeremić, K., Nedeljković, J.M., 2007, In Situ Radical Polymerization Of Methyl Methacrylate in a Solution of Surface Modified TiO2 and Nanoparticles., European Polymer Journal, 43, 3719-3726 |
| [9] | Wu, G., Gan, S., Cui, L., Xu, Y., 2008, Preparation and Characterization of PES/TiO2 Composite Membranes., Applied Surface Science., 254, 7080-7086 |
| [10] | Chatterjee, A., and Islam, M.S., 2008, Fabrication and Characterization of TiO2-Epoxy Nanocomposite., Materials Science and Engineering A, 487, 574-585 |
| [11] | Zhao, Y., Li, C., Liu, X., Gu, F., Jiang, H., Shao, W., Zhang, L., He, Y., 2007, Synthesis and Optical Properties of TiO2 Nanoparticles., Materials Letters, 61, 79-83 |
| [12] | Delgado, A., and Matijevic, E., 1991, Particle Size Distribution of Inorganic Colloidal Dispersions: A Comparison of Different Techniques., Part. Part. Syst. Charact., 8, 128-135 |
| [13] | Pérez-Rodríguez, J.L., Carrera, F., Poyato, J., Pérez-Maqueda, L.A., 2002, Sonication as a Tool for Preparing Nanometric Vermiculite Particles., Nanotechnology, 13, 382-387 |
| [14] | Franco, F., Pérez-Maqueda, L.A., Pérez-Rodríguez, J.L., 2004, The Effect of Ultrasound on the Particle Size and Structural Disorder of a Well-Ordered Kaolinite., Journal of Colloid and Interface Science, 274, 107-117 |
| [15] | Franco, F., Pérez-Maqueda, L.A., Pérez-Rodríguez, J.L., 2004, Influence of the Particle-Size Reduction by Ultrasound Treatment on the Dehydroxylation Process of Kaolinites., Journal of Thermal Analysis and Calorimetry., 78, 1043-1055 |
| [16] | Pérez-Maqueda, L.A., Duran, A., Pérez-Rodríguez, J.L., 2005, Preparation of Submicron Talc Particles by Sonication., Applied Clay Science., 28, 245-255 |
| [17] | Fu, S.-Y., Feng, X.-Q., Lauke, B. Mai, Y.-W., 2008, Effects of Particle Size, Particle/Matrix Interface Adhesion and Particle Loading on Mechanical Properties of Particulate- Polymer Composites., Composites, Part B 39, 933-961 |
| [18] | Zhu, Z.K., Yang, Y., Yin, J. Qi, Z.N., 1999, Preparation and Properties of Organosoluble Polyimide/Silica Hybrid Materials by Sol-Gel Process., J Appl Polym Sci., 73, 2977-2984. |
| [19] | Dekkers, M.E.J., and Heikens, D., 1983, The Effect of Interfacial Adhesion on the Tensile Behavior of Polystyrene-Glass-Bead Composites., J Appl Polym Sci., 28, 3809-3815 |
| [20] | Fu, S.Y., and Lauke, B., 1998, Characterization of Tensile Behaviour of Hybrid Short Glass Fibre Calcite Particle ABS Composites., Composite Part A, 29A, 573-583 |
| [21] | Fu, S.Y., and Lauke, B., 1997, Analysis of Mechanical Properties of Injection Short Glass Fibre (SGF)/Calcite/ABS Composites., J Mater Sci Technol., 13, 389-396 |
| [22] | Eirich, F. R., 1984, Some Mechanical and Molecular Aspects of the Performance of Composites., J Appl Polym Sci Appl Polym Symp, 39, 93-102 |
| [23] | Radford, K.C., 1971, The Mechanical Properties of an Epoxy Resin with a Second Phase Dispersion., J Mater Sci., 6, 1286-1291 |
| [24] | Spanoudakis, J., and Young, R.J., 1984, Crack Propagation in a Glass Particle-Filled Epoxy-Resin. 1. Effect of Particle-Volume Fraction and Size., J Mater Sci., 19, 473-486 |
| [25] | Amdouni, N., Sautereau, H. Gerard, J.F., 1992, Epoxy Composites Based on Glass-Beats. 2. Mechanical-properties., J Appl Polym Sci., 46, 1723-1735 |
| [26] | Wang, M., Berry, C., Braden, M. Bonfield, W., 1998, Young’s and Shear Moduli of Ceramic Particle Filled Polyethylene., J Mater Sci Mater Med., 9, 621-624 |
| [27] | Ou, Y., Yang, F., Yu, Z.Z., 1998, A new conception on the toughness of nylon 6/silica nanocomposite prepared via in situ polymerization., J Polym Sci Part B Polym Phys., 36, 789-795 |
| [28] | Xie, X.L., Zhou, X.P. Mai, Y-W., 2005, Dispersion and Alignment of Carbon Nanotubes In Polymer Matrix: A Review., Mater Sci Eng R., 49, 89-112 |
| [29] | Nakamura, Y., Yamaguchi, M., Okubo, M. Matsumoto, T., 1992, Effect of Particle Size on Mechanical Properties of Epoxy Resin Filled with Angular-Shaped Silica., J Appl Polym Sci., 44, 151-158 |
| [30] | Zhang, Q, Tian, M., Wu, Y., Lin, G. Zhang, L, 2004, Effect of Particle Size on the Properties of Mg (OH) 2-Filled Rubber Composites., J Appl Polym Sci., 94, 2341-2346 |
| [31] | Lazzeri, A., Thio, Y.S., Cohen, R.E., 2004, Volume Strain Measurements on CaCO3/Polypropylene Particulate Composites: The Effect of Particle Size., J Appl Polym Sci., 91, 925-935 |
| [32] | Suprapakorn, N., and Dhamrongvaraporn, S.S., 1998, Effect of CaCO3 on the Mechanical and Rheological Properties of a Ring-Opening Phenolic Resin: Polybenzoxazine., Polym Compos., 19, 126-132 |
| [33] | Singh, R.P., Zhang, M., Chan, D., 2002, Toughening of a Brittle Thermosetting Polymer: Effects of Reinforcement Particle Size and Volume Fraction., J Mater Sci., 37, 781-788 |
| [34] | Lange, F.F., and Radford, K.C., 1971, Fracture Energy of an Epoxy Composite System., J Mater Sci., 6, 1197-1203 |
| [35] | Ji, X.L., Jing, J.K., Jiang, B.Z., 2002, Tensile Modulus of Polymer Nanocomposites., Polym Eng Sci., 42, 983-993 |
| [36] | Mishra, S., Sonawane, S.H. Singh, R.P., 2005, Studies on Characterization of Nano Caco3 Prepared by the In Situ Deposition Technique and its Application in PP-Nano CaCO3 Composites., J Polym Sci Part B Polym Phys., 43, 107-113 |
| [37] | Douce, J., Boilot, J.P., Biteau, J., Scodellaro, L. Jimenez, A., 2004, Effect of Filler Size and Surface Condition of Nano-Sized Silica Particles in Polysiloxane Coatings., Thin Solid Films, 466, 114-122 |
| [38] | Sahu, S., and Broutman, L.J., 1972, Mechanical Properties of Particulate Composites., Polym Eng Sci., 12, 91-100 |
| [39] | Erdem, B., Hunisicker, R.A., Simmons, G.W., Sudol, E.D., Dimonie, V.L. El-Aasser, M.S., 2001,. XPS and FTIR Surface Characterization of TiO2 Particles Used in Polymer Encapsulation., Langmuir, 17, 2664-2669 |
| [40] | Murariu, M., Ferreira, A., Da, S., Degée, P., Alexandre, M., Dubois, P., 2007, Polylactide Compositions. Part 1: Effect of Filler Content and Size on Mechanical Properties of PLA/Calcium Sulfate Composites., Polymer, 48, 2613-2618 |
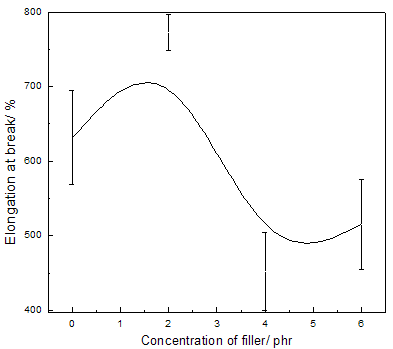
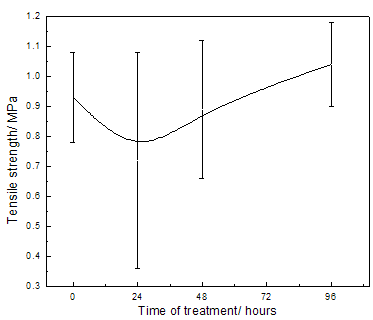
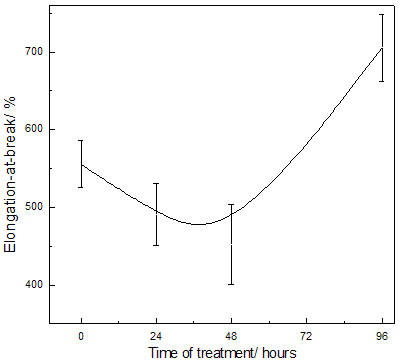
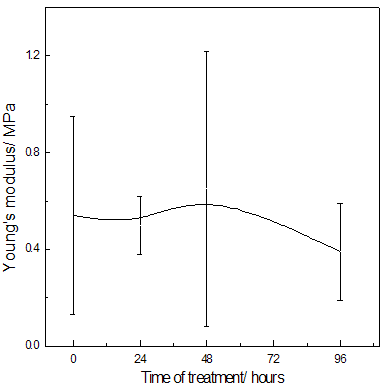
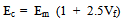
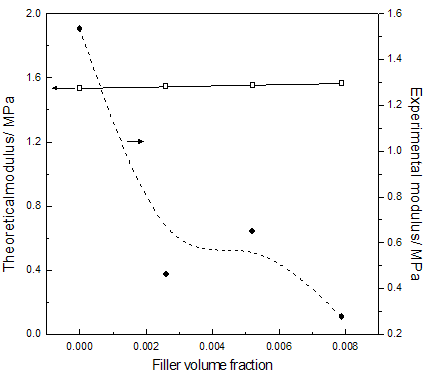
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML