Asif Sakib1, Tarikul Islam1, Mafzal Ahmed1, Md. Rezaul Karim1, Md. Rasel Hossen2
1Department of Textile Engineering, Port City International University, Chittagong, Bangladesh
2Department of Apparel Engineering, Bangladesh University of Textiles, Dhaka, Bangladesh
Correspondence to: Tarikul Islam, Department of Textile Engineering, Port City International University, Chittagong, Bangladesh.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This paper aims to scrutinize the change in physical and chemical properties of cotton knitted fabric with a variety of shade depth (1% to 8%) dyeing with reactive dyes. Shade percentage is an important factor which has various impacts on 100% cotton knitted fabric. The technical factors of GSM, color fastness to rubbing, bursting strength test, color fastness to wash, and color fastness to perspiration of cotton knitted fabric are influenced by various shade percentage (1% to 8%). The cotton knitted bleached fabric which was dyed with Remazol Red RR reactive dye and auxiliaries with different shade percentage, then measured GSM & their fastness properties. It was observed that after increasing the shade percentage on cotton knitted fabric, GSM was increased but bursting strength is decreased. On the other hand, color fastness to rubbing, wash and perspiration is decreased repeatedly.
Keywords:
Color Fastness, GSM, Knitted Fabric, Reactive Dye, Shade Variation
Cite this paper: Asif Sakib, Tarikul Islam, Mafzal Ahmed, Md. Rezaul Karim, Md. Rasel Hossen, A Comparative Study on Effect of Shade Depth on Various Properties of Cotton Knitted Fabric Dyed with Reactive Dyes, International Journal of Clothing Science, Vol. 4 No. 1, 2017, pp. 12-16. doi: 10.5923/j.clothing.20170401.02.
1. Introduction
The use of knitted fabric has been rapidly increased worldwide. Both men & women feel comfortable wearing knitted fabric for their shape fitting properties, softer handle, bulkier nature and high extension at low tension compared to woven fabric [1].Cotton today is the most used textile fiber in the world. Its current market share is about 56% for all fibers used for apparel and home furnishings and sold in the U.S. Another contribution is attributed to nonwoven textiles and personal care items. It is generally recognized that most consumers prefer cotton personal care items to those containing synthetic fibers. Current estimates for world productions are about 25 million tones or 110 million bales annually, accounting for 2.5% of the world's arable land. China is the world's largest producer of cotton, but most of this is used domestically. The United States has been the largest exporter for many years [2].In recent years, reactive dyes have been most commonly used and the reactive dyes are the best for cotton for its wide range of application and better fastness properties [3]. There for 50% of cellulosic fibers are dyed with reactive dyes. Share of reactive dyes among all textile dyes is 29%. Due to their strong interaction with many surfaces of synthetic and natural fabrics, reactive dyes are used for dyeing wool, cotton, nylon, silk, and modified acrylics [4, 5]. In Bangladeshi wet processing industries, reactive dyes are hugely used. The reactive site of the dyes reacts with functional group on fiber under influence of heat and alkali [6]. Fiber reactive dyes react with the cellulosic fiber in the presence of alkali to form a strong covalent chemical bond between a carbon atom of the dye molecule and an oxygen atom of the hydroxyl group in the cellulose. Reactive dyes typically form covalent linkages between the dye and the substrate when subjected to the proper conditions. Reactive dyes are popular in textile manufacturing due to their fastness properties.In this research we used cotton weft knitted (single jersey) fabric & reactive dyes. All of the samples were dyed by reactive dyes (Remazol Red RR), and then GSM, bursting strength, & various color fastness properties were measured.
2. Materials and Methods
2.1. Materials
2.1.1. Sample Preparation
In this research various weft knitted fabric samples were prepared from Divine Fabrics Ltd, Gazipur, Bangladesh. These fabrics were 100% cotton knitted single jersey and it was made from cotton yarn, GSM about in average was 160.
2.1.2. Dye Stuff and Chemicals
The chemicals and dye stuff were collected from Antim Knit Composite Ltd, Narayangonj, Bangladesh and used without any further treatmenti. Reactive Dye (Remazol Red RR)ii. Electrolyte: Glauber salt (Na2SO4. 10 H2O)iii. Alkali: Soda ash and caustic iv. Soaping agent (SW CONE)v. Acetic Acid (100%)
2.2. Methods
2.2.1. Dyeing of Samples with Reactive Dyes
With a view to performing this research, we dyed the samples by variation of 1% to 8% shade of reactive dye. Dyeing parameters of different shade% are applied on samples.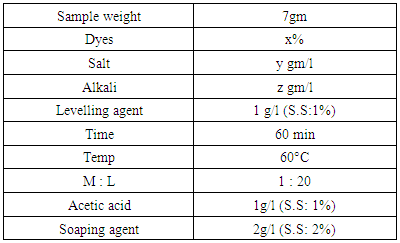
2.2.2. GSM and Bursting strength Properties
GSM (gram per square meter) of dyed weft knitted fabric was evaluated for respective shade percentage and then compared. Bursting strength test is measured by according to ASTM D3786 test method.
2.2.3. Fastness Measurement
In this research, the following fastness properties were measured [7, 9].• Color fastness to wash (ISO 105 C04 B2S)• Color fastness to rubbing (ISO 105 X12)• Color fastness to perspiration (ISO 105 E04)The dyeing of samples carried out by using exhaust brand reactive dyes on lab dyeing machine keeping material to liquor ratio 1:20 for the shade percentage 1% to 8%. All dyeing operations were performed as per the standard method prescribed by the manufacturers. The pH of the dye bath was adjusted with 20 g/l soda ash. At first 10 dyeing pots were marked for the 10 samples. Set the bath with substrate at room temperature 40°C and add sample, dyes soda ash and salt. Then raise the temperature at 60°C at 3°/minute. Run the dyeing for 60 minutes at as same temperature 60°C. Decrease the temperature from 60°C to room temperature. Then dropped the samples from bath and rinsed and then carried on after treatment process. After dyeing the samples washed by hot water with S.W. Cone (detergent) & rinsed. Then the samples were washed with cold water & neutralized by 1g/l acetic acid (100%) for 10 minutes. Dry the sample by incubator (dryer).GSM of fabrics were measured with the help of GSM cutter & electric balance. Then the color fastness to wash, rubbing, perspiration and were measured by ISO 105 C04 B2S, ISO 105 X12 and ISO 105 E04 test method respectively [7-9].
3. Results and Discussion
All the tests were performed in the standard testing atmosphere i.e. 65±2% R.H. and 20°C. Ten sample of each shade of weft knitted fabric samples (single jersey) were taken for this experiment. The results of different test of different samples are given below.
3.1. Analysis of GSM and Bursting Strength
3.1.1. Areal Density (GSM) of Samples for Different Shade Percentage
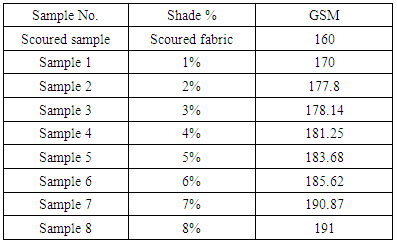
GSM Test:Table 1. GSM Effect according to color depth
 |
| |
|
3.1.2. Bursting Strength Test
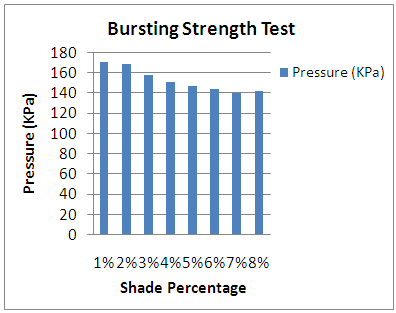
Bursting Strength of samples according to different shade percentage (%).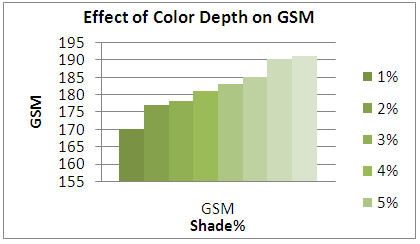 | Figure 1. GSM effect according to shade variation |
Table 2. Effect of Bursting Strength Test according to shade depth
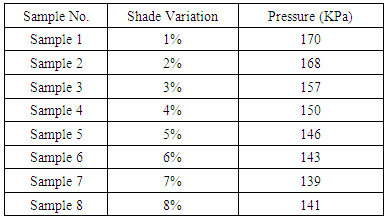 |
| |
|
 | Figure 2. Effect of Bursting Strength Test |
From the above figure of our test report we find that with the increasing of colour shade variation (1% to 8%), due to amount of dye particle interact and put together with fabric G.S.M of dyed sample is increased but on the other side with increase the amount of alkali (soda ash+caustic) which is interact with cotton yarn, is accountable to loss fabric strength resulting decreased in bursting strength is.
3.2. Analysis of Fastness Properties
3.2.1. Color Fatness to Rubbing
Effect of Color Fastness to Rubbing according to shade depth.Table 3. Effect of Color Fastness to Rubbing
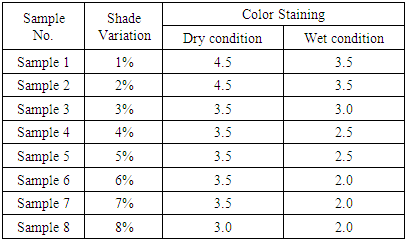 |
| |
|
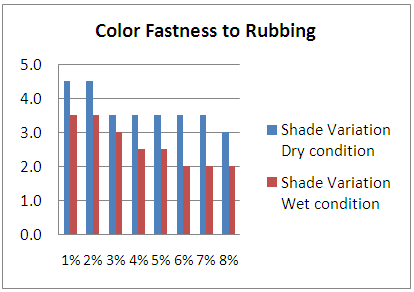 | Figure 3. Effect of Color Fastness to Rubbing |
3.2.2. Color Fatness to Wash
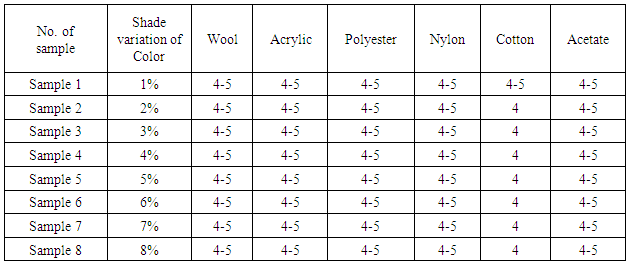
The result of color fastness to wash according to different shade depth is given below:Table 4. Effect of Colour Fastness to Washing
 |
| |
|
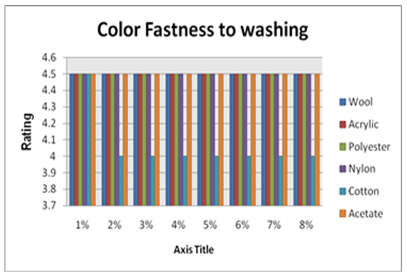 | Figure 4. Color Fastness to Washing |
From the figure, here we got significant change on color fastness to rubbing and washing according to acid and alkali based solution for different solution. With the increase of shade depth color fastness to rubbing and washing was changed on color and also its staining was increased.
3.2.3. Colour Fastness to Perspiration (Acid)
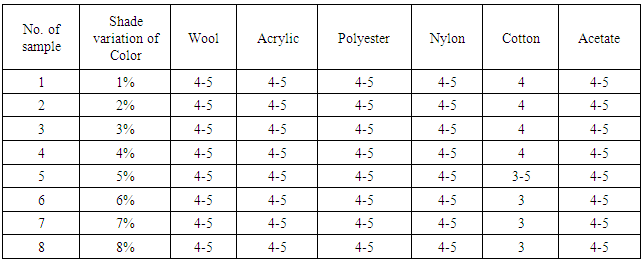
Table 5. Effect of Colour Fastness to Perspiration (Acid)
 |
| |
|
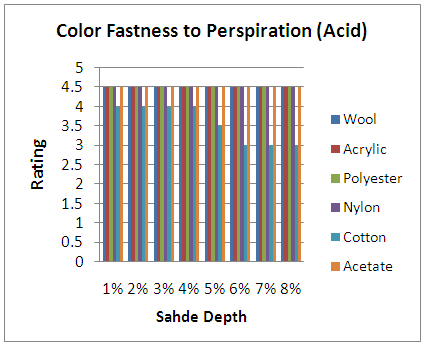 | Figure 5. Color Fastness to Perspiration (Acid) |
3.2.4. Color Fastness to Perspiration (Alkali)
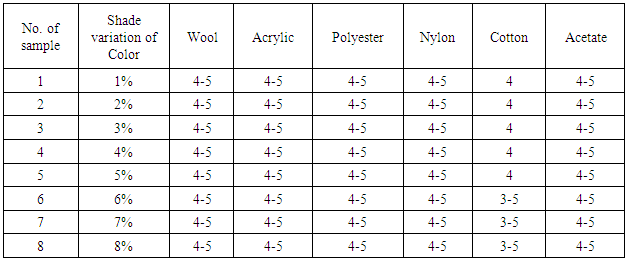
Table 6. Effect of Color Fastness to Perspiration (Alkali)
 |
| |
|
 | Figure 6. Color Fastness to Perspiration (Alkali) |
From the figure, here we got significant change on color fastness to perspiration was decrease on color shade depth (1% to 8%) when dye molecules arrest the threads at the interlacement positions. It’s staining is increased according to acid and alkali based solution.
4. Conclusions
In this study, it was observed that, with the increase of color shade depth (1% to 8%), the structural properties of cotton knitted fabric according to different acid and alkali based solution. that with the increasing of color shade variation (1% to 8%), due to amount of dye particles’ interaction and put together with fabric G.S.M of dyed sample is increased but on the other side with increase the amount of alkali (soda ash+casutic) which interacts with cotton yarn, is accountable to loss fabric strength resulting decreased in bursting strength. In another side, with color fastness of weft knitted fabric is also affected by shade. With the increase of shade (1% to 8%) color fastness to wash, rubbing and perspiration of cotton knitted fabric according to different acid and alkali based solution is decreased. So it is said easily comment that, there is a considerable effect of dye shade variation (1% to 8%) cotton knitted fabric with reactive dyes (Remazol Red RR).
ACKNOWLEDGEMENTS
The authors would like to thank to Md. Abdullah Al Mamun, Assistant professor, Dept. of Textile Engineering, MBSTU, Bangladesh. Also thanks to Md. Rafikul Islam, Dyeing Lab Assistant Bureau Varitas Consumer Product Limited, Dhaka for their good support, help and coordination at all points during our data collection.
References
| [1] | N., Anbumani, Knitting Fundamentals, Machine, Structures and Developments, First Edition, New Age International Publishers, New Delhi, 2007, 274pp. |
| [2] | http://en.wikipedia.org/wiki/Cotton. |
| [3] | Ramasamy, M. and Kandasaamy, P. V, 2005, Effect of cationization of cotton on its dyeability, Indian Journal of Fibre & Textile Research, 30(3), 315-323. |
| [4] | Mottaleb, M., and Littlejohn, D., 2001, Application of an HPLCeFTIR modified thermospray interface for analysis of dye samples, US National Library of Medicine National Institutes of Health, 17(3), 429-434. |
| [5] | Al-Degs, Y.S., El-Barghouthi, M.I., Khraisheh, M.A., Ahmad, M.N., and Allen, S.J., 2004, Effect of surface area, Micropores, secondary micropores and mesopores volumes of activated carbons on reactive dyes adsorption from solutionon, Separation Science and Technology, 39(1), 97-111. |
| [6] | Saeed, Q., I.A. Bhatti, M. Zuber, S. Nosheen, M.A. Zia and M. Abbas., 2013, “Study of application of mono azo reactive dyes on cotton by exhaust method and printing properties”, International Journal of Basic & Applied Sciences, 12(6), 191-197. |
| [7] | ISO 105-C04:1989; Textiles Tests for colour fastness- Part C04: Color fastness to washing: Test 4. |
| [8] | ISO 105-X12:2001; Textiles Tests for color fastness– Part X12: Color fastness to rubbing. |
| [9] | ISO 105 E04:1994; Textiles Tests for color fastness- Part B02: Color fastness to perspiration. |









 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML




