-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2025; 15(3): 59-63
doi:10.5923/j.chemistry.20251503.01
Received: Apr. 30, 2025; Accepted: May 23, 2025; Published: Jun. 13, 2025

Harnessing Ionic Liquids for Rapid Alkylation: Efficient Access to Methyl 2-oxo-hexahydro-2H-cyclopenta[b]furan-3-carboxylate
Bello Makama
The Department of Chemistry and Physical Sciences, Nicholls State University, 906 East First Street, Thibodaux, Louisiana 70310, United States of America
Correspondence to: Bello Makama , The Department of Chemistry and Physical Sciences, Nicholls State University, 906 East First Street, Thibodaux, Louisiana 70310, United States of America.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

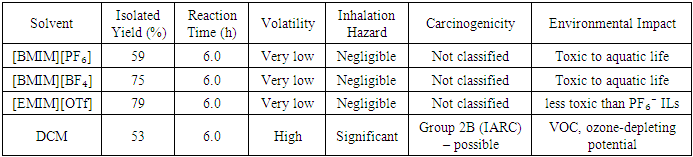
An efficient synthetic protocol for the alkylation of 2-chlorocyclopentanone with dimethyl malonate was developed using sodium hydride as the base and various ionic liquids as reaction media. This study evaluated three ionic liquids—[BMIM]PF₆, [BMIM]BF₄, and [EMIM]PF₆—and compared their performance directly to that of a conventional DCM solvent. Under optimized conditions (70°C, 6 h), the ionic liquids achieved significantly enhanced yields of dimethyl 2-(2-oxocyclopentyl) malonate: [BMIM]PF₆ provided a yield of 59%, [BMIM]BF₄ yielded 75%, and [EMIM]OTf reached 79%, in contrast to the 53% yield observed with the DCM system. The superior performance of the ionic liquids is attributed to their high polarity and unique solvation properties, which promote efficient deprotonation of dimethyl malonate and stabilize the reactive enolate intermediate, thereby lowering the activation barrier for the nucleophilic substitution step. These findings underscore the potential of ionic liquids as sustainable and efficient alternatives to traditional organic solvents, offering improved reaction kinetics and enhanced yields for carbon–carbon bond-forming reactions. The methodology presented herein contributes to the advancement of greener synthetic strategies in organic chemistry.
Keywords: Ionic liquids, Alkylation, 2-Chlorocyclopentanone, Dimethyl malonate, Enolate formation, Nucleophilic substitution, Green chemistry, Sustainable synthesis
Cite this paper: Bello Makama , Harnessing Ionic Liquids for Rapid Alkylation: Efficient Access to Methyl 2-oxo-hexahydro-2H-cyclopenta[b]furan-3-carboxylate, American Journal of Chemistry, Vol. 15 No. 3, 2025, pp. 59-63. doi: 10.5923/j.chemistry.20251503.01.
Article Outline
1. Introduction
- Ionic liquids (ILs) have garnered substantial interest as alternative media in organic synthesis, attributed to their unique physicochemical properties, which include negligible vapor pressure, high thermal stability, and tunable solvation characteristics. [1] These distinctive features render ILs particularly appealing as green solvents in synthesis and catalysis. [2,3] Initially derived from molten salts, ILs have developed into a diverse class of room-temperature solvents that are suitable for a wide array of industrial and academic applications. [4] Their classification as hydrophilic or hydrophobic, along with their structural tunability, further extends their applicability in green chemistry. [5,6]The versatility of ILs encompasses stereoselective and asymmetric synthesis. [7] Their negligible volatility and high chemical stability facilitate their use under demanding reaction conditions. [8] In the realm of organic synthesis, ILs have demonstrated effectiveness across numerous transformations, ranging from C–C bond formation to complex cyclization reactions. [9,10] This adaptability is attributable to the ability to tailor both the cation and anion, thus fine-tuning solvation characteristics and reactivity profiles. [11,12]Quantitative structure–activity relationships have been utilized to predict the toxicity of ILs, emphasizing the necessity of designing task-specific ILs with favorable environmental and biological profiles. [13,14] Moreover, ILs have achieved notable success in catalysis, including applications involving both Brønsted and Lewis acid sites. [15,16] In chemical manufacturing and processing, they contribute to waste minimization, recyclability, and overall sustainability. [17,18]A particularly advantageous aspect of ILs is their dual functionality, enabling them to serve as both solvent and catalyst, often eliminating the need for additional reagents. [19] For instance, efficient alkylation of enolate intermediates has been accomplished in ILs, frequently surpassing traditional solvent systems in terms of both selectivity and yield. [20,21] ILs are especially appropriate for reactions that require high ion stabilization, exemplified by the alkylation of 2-chlorocyclopentanone with active methylene compounds. [22]Despite the well-documented advantages of ILs, comprehensive side-by-side comparisons with conventional solvents remain limited, particularly for challenging transformations involving steric hindrance or competing side reactions. [23] Previous research by Makama [24] demonstrated that the alkylation of 2-chlorocyclopentanone with dimethyl malonate in ILs yielded diesters with favorable stereochemistry and clean spectral profiles, as verified by NMR, IR, and MS techniques.In addition to traditional reactions, ILs are increasingly being utilized in biocatalysis, where their unique solvation environments can enhance enzyme stability and activity. [25] Concurrently, structurally similar deep eutectic solvents (DESs) have emerged as promising green alternatives, providing comparable physicochemical benefits at a lower cost. [26] ILs have also proven effective in microwave-assisted organic synthesis, which allows for rapid reaction acceleration under environmentally benign conditions. [27]Consequently, ILs represent a compelling platform for innovation in synthetic chemistry. Their environmental compatibility, tunable properties, and catalytic potential render them ideal for integration into modern, sustainable methodologies. The present study evaluates the use of [BMIM]PF₆, [BMIM]BF₄, and [EMIM]PF₆ in the alkylation of 2-chlorocyclopentanone with dimethyl malonate, comparing their performance to that of traditional THF/DCM systems under identical reaction conditions. The objective is to benchmark IL-based processes not only by yield and selectivity but also by their broader environmental and mechanistic advantages.
2. Results and Discussion
- It has been previously demonstrated that 2-chlorocyclopentanone (1) can be converted into dimethyl 2-(2-oxocyclopentyl) malonate (2), resulting in the formation of the corresponding diester (2) with a yield of 53%. We reported earlier that the ¹H NMR spectrum provided substantial evidence supporting the proposed structure (2), displaying a doublet centered at δ 3.84 ppm (J = 5.8 Hz), which corresponds to a methine proton associated with the diester. Furthermore, two singlets, each integrating for three protons, were identified at δ 3.78 ppm and δ 3.73 ppm, representing the methyl ester protons. A one-proton resonance at δ 2.97–2.88 ppm was assigned to the methine proton adjacent to the ketone. The presence of the ester groups and the ketone was further validated by the IR spectrum, which exhibited characteristic absorptions at 1738 cm⁻¹ and 1749 cm⁻¹, respectively. Additionally, the mass spectrum corroborated these findings, revealing a molecular ion at m/z 214 (Scheme 1). [24]
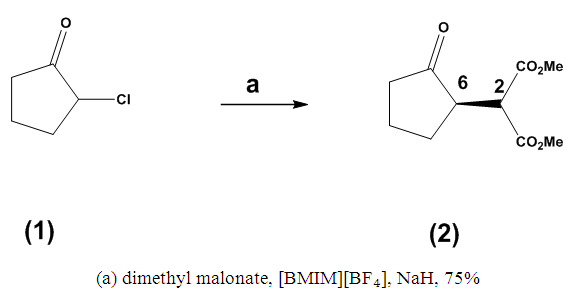 | Scheme 1. [BMIM][BF₄], Mediated Alkylation |
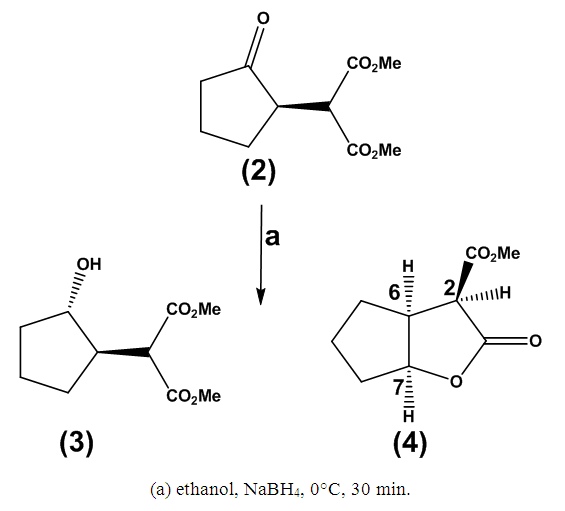 | Scheme 2. Lactonization Mediated by Sodium Borohydride |
|
3. Experimental Techniques
- Commercial reagents were procured from Thermo Scientific or VWR and were utilized either as received or subjected to purification prior to application, adhering to established protocols. Non-aqueous reagents were transferred under a nitrogen atmosphere utilizing a syringe. Organic solutions were concentrated under reduced pressure employing a Büchi rotary evaporator with a water bath, or they were evaporated using a sand bath. Thin-layer chromatography (TLC) was conducted on Merck aluminum-backed plates coated with 0.2 mm silica gel 60-F. Visualization of the developed chromatogram was achieved through UV fluorescence quenching at 254 nm.Proton (¹H) and carbon (¹³C) nuclear magnetic resonance (NMR) spectra were acquired on a Jeol JNM-ECZ spectrometer operating at 400 MHz for protons. Data for ¹H NMR are reported as follows: chemical shift (δ, ppm), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), integration, and coupling constant (Hz). Data for ¹³C NMR spectra are presented in terms of chemical shift (ppm) relative to tetramethylsilane (TMS). Infrared (IR) spectra were recorded on a Bruker Alpha spectrometer utilizing direct attenuated total reflectance (ATR) methodology. All absorption frequencies are reported in units of cm⁻¹.
3.1. Experimental
- Dimethyl 2-(2-oxocyclopentyl)malonate (2)
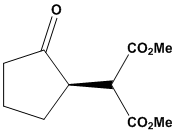 Method ATo a stirred solution of sodium hydride (158 mg, 6.58 mmol, 1.30 equivalents) in dichloromethane (10 mL) maintained at 0°C, dimethyl malonate (1.05 mg, 6.58 mmol, 1.3 equivalents) was added. The resulting solution was stirred for 30 minutes at ambient temperature. Subsequently, 2-chlorocyclopentanone (1) (600 mg, 5.06 mmol, 1 equivalent) was introduced, and the mixture was stirred at ambient temperature for an additional 8 hours. Thin-layer chromatography (TLC) analysis at this stage indicated the formation of a new product. To the reaction mixture, saturated aqueous ammonium chloride solution (10 mL) was added, and the organic layer was extracted with diethyl ether (4 × 15 mL). The combined organic layers were washed with saturated sodium bicarbonate solution (4 × 10 mL) and brine (4 × 10 mL), then dried over magnesium sulfate and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel, utilizing a gradient of solvents from hexane:ethyl acetate (5:1 to 100% ethyl acetate), yielding the title compound as a colorless oil (430 mg, 41% yield). The product was characterized using spectroscopic data: υmax (thin film/cm-1) 2961, 1749, 1738; δH (250 MHz, CDCl3) 3.84 (1H, d, J 5.8 Hz, CH(CO2CH3)2), 3.78 (3H, s, CH3), 3.73 (3H, s, CH3), 2.97-2.88 (1H, m, CHC=O), 2.56-1.99 (4H, m, CH2CH, CH2C=O), 2.00-1.81 (2H, m, CH2CH2C=O); δC (62.5 MHz, CDCl3) 216.2, 169.1, 168.6, 53.9, 51.6, 51.2, 41.2, 38.2, 23.8, 20.8; m/z (C.I) 215 (MH+, 100%), 214 (4%), 183 (11%), 155 (7%), 123 (3%); C10H15O5, requires 215.092, found: 215.0913.Method BTo a stirred solution of sodium hydride (158 mg, 6.58 mmol, 1.30 equivalents) in dichloromethane (10 mL) at 0°C, dimethyl malonate (1.05 mg, 6.58 mmol, 1.3 equivalents) was added. The resulting solution was stirred for 30 minutes at room temperature. Subsequently, 2-chlorocyclopentanone (1) (600 mg, 5.06 mmol, 1 equivalent) was introduced to the solution and the mixture was stirred at room temperature for an additional 8 hours. Thin-layer chromatography (TLC) analysis at this point indicated the formation of a new product. To the reaction mixture, saturated aqueous ammonium chloride solution (10 mL) was added, and the organic layer was extracted with diethyl ether (4 x 15 mL). The combined organic layers were washed with saturated sodium bicarbonate solution (4 x 10 mL) and brine (4 x 10 mL), then dried over magnesium sulfate and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel, employing a gradient of solvents from hexane: ethyl acetate (5:1 to 100% ethyl acetate), yielding the title compound as a colorless oil (734 mg, 68% yield).Dimethyl 2-2-hydroxycyclopentyl) malonate (3)Methyl 2-oxo-hexahydro-2H-cyclopenta[b]furan-3-carboxylate (4)
Method ATo a stirred solution of sodium hydride (158 mg, 6.58 mmol, 1.30 equivalents) in dichloromethane (10 mL) maintained at 0°C, dimethyl malonate (1.05 mg, 6.58 mmol, 1.3 equivalents) was added. The resulting solution was stirred for 30 minutes at ambient temperature. Subsequently, 2-chlorocyclopentanone (1) (600 mg, 5.06 mmol, 1 equivalent) was introduced, and the mixture was stirred at ambient temperature for an additional 8 hours. Thin-layer chromatography (TLC) analysis at this stage indicated the formation of a new product. To the reaction mixture, saturated aqueous ammonium chloride solution (10 mL) was added, and the organic layer was extracted with diethyl ether (4 × 15 mL). The combined organic layers were washed with saturated sodium bicarbonate solution (4 × 10 mL) and brine (4 × 10 mL), then dried over magnesium sulfate and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel, utilizing a gradient of solvents from hexane:ethyl acetate (5:1 to 100% ethyl acetate), yielding the title compound as a colorless oil (430 mg, 41% yield). The product was characterized using spectroscopic data: υmax (thin film/cm-1) 2961, 1749, 1738; δH (250 MHz, CDCl3) 3.84 (1H, d, J 5.8 Hz, CH(CO2CH3)2), 3.78 (3H, s, CH3), 3.73 (3H, s, CH3), 2.97-2.88 (1H, m, CHC=O), 2.56-1.99 (4H, m, CH2CH, CH2C=O), 2.00-1.81 (2H, m, CH2CH2C=O); δC (62.5 MHz, CDCl3) 216.2, 169.1, 168.6, 53.9, 51.6, 51.2, 41.2, 38.2, 23.8, 20.8; m/z (C.I) 215 (MH+, 100%), 214 (4%), 183 (11%), 155 (7%), 123 (3%); C10H15O5, requires 215.092, found: 215.0913.Method BTo a stirred solution of sodium hydride (158 mg, 6.58 mmol, 1.30 equivalents) in dichloromethane (10 mL) at 0°C, dimethyl malonate (1.05 mg, 6.58 mmol, 1.3 equivalents) was added. The resulting solution was stirred for 30 minutes at room temperature. Subsequently, 2-chlorocyclopentanone (1) (600 mg, 5.06 mmol, 1 equivalent) was introduced to the solution and the mixture was stirred at room temperature for an additional 8 hours. Thin-layer chromatography (TLC) analysis at this point indicated the formation of a new product. To the reaction mixture, saturated aqueous ammonium chloride solution (10 mL) was added, and the organic layer was extracted with diethyl ether (4 x 15 mL). The combined organic layers were washed with saturated sodium bicarbonate solution (4 x 10 mL) and brine (4 x 10 mL), then dried over magnesium sulfate and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel, employing a gradient of solvents from hexane: ethyl acetate (5:1 to 100% ethyl acetate), yielding the title compound as a colorless oil (734 mg, 68% yield).Dimethyl 2-2-hydroxycyclopentyl) malonate (3)Methyl 2-oxo-hexahydro-2H-cyclopenta[b]furan-3-carboxylate (4)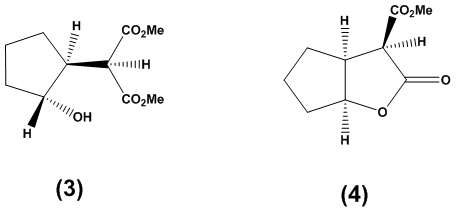 To a stirred solution of dimethyl 2-(2-oxocyclopentyl) malonate (2) (80 mg, 0.37 mmol, 1.00 equiv) in ethanol (5 mL) contained within an Erlenmeyer flask, sodium borohydride (NaBH4) was added in small portions at room temperature (16.8 mg, 0.44 mmol, 1.20 equiv) over a period of 15 minutes. The reaction mixture was subsequently stirred for an additional 30 minutes and then transferred to water (5 mL). A 5% dilute solution of hydrochloric acid (4 drops) was introduced, and the organic layer was extracted with diethyl ether (2 x 10 mL), dried over magnesium sulfate (MgSO4), and concentrated under reduced pressure. Column chromatography on silica gel, eluting with a mixture of hexane and ether (3:1), yielded product (3) as a colorless oil (66 mg, 65% yield). The spectral data obtained included: υmax (thin film/cm-1) 3504, 2955, 2255, 1731; δH (250 MHz, CDCl3) 3.96-3.91 (1H, m, CHOH), 3.68 (3H, s, CH3), 3.67 (3H, s, CH3), 3.32 (1H, d, J7.8 Hz, CH(CO2Me)2), 2.33-2.28 (1H, m, CH), 1.88-1.83 (2H, m, CH2), 1.63-1.53 (3H, m, CH2), 1.28-1.25 (1H, m, CH2); δC (62.5 MHz, CDCl3) 169.2, 168.6, 76.2, 55.9, 51.5, 51.0, 46.8, 33.4, 28.3, 21.3;m/z (C.I) 216 (MH+, 9%), 159 (10%), 150 (4%), corresponding to C10H17O5, with a calculated mass of 216.0998 and a found mass of 216.0935. Further elution provided product (4) (18 mg, 26% yield), with the following spectral data: υmax (thin film/cm-1) 2957, 1742, 1733; δH (250 MHz, CDCl3) 5.06 (1H, t, J 5.3 Hz, CHO), 3.73 (3H, s, CO2CH3), 3.68 (1H, d, J 3.3 Hz, CHC=O), 3.14-3.10 (1H, m, CHCHC=O), 1.98-1.56 (6H, m, CH2); δC (62.5 MHz, CDCl3) 176.9, 168.5, 86.5, 54.8, 53.7, 43.5, 33.5, 32.9, 23.9;m/z (C.I) 185 (MH+, 45%), 141 (51%), 134 (43%), 124 (67%), 101 (77%), 84 (54%) corresponding to C9H13O4, with a calculated mass of 185.0814 and a found mass of 185.0806.
To a stirred solution of dimethyl 2-(2-oxocyclopentyl) malonate (2) (80 mg, 0.37 mmol, 1.00 equiv) in ethanol (5 mL) contained within an Erlenmeyer flask, sodium borohydride (NaBH4) was added in small portions at room temperature (16.8 mg, 0.44 mmol, 1.20 equiv) over a period of 15 minutes. The reaction mixture was subsequently stirred for an additional 30 minutes and then transferred to water (5 mL). A 5% dilute solution of hydrochloric acid (4 drops) was introduced, and the organic layer was extracted with diethyl ether (2 x 10 mL), dried over magnesium sulfate (MgSO4), and concentrated under reduced pressure. Column chromatography on silica gel, eluting with a mixture of hexane and ether (3:1), yielded product (3) as a colorless oil (66 mg, 65% yield). The spectral data obtained included: υmax (thin film/cm-1) 3504, 2955, 2255, 1731; δH (250 MHz, CDCl3) 3.96-3.91 (1H, m, CHOH), 3.68 (3H, s, CH3), 3.67 (3H, s, CH3), 3.32 (1H, d, J7.8 Hz, CH(CO2Me)2), 2.33-2.28 (1H, m, CH), 1.88-1.83 (2H, m, CH2), 1.63-1.53 (3H, m, CH2), 1.28-1.25 (1H, m, CH2); δC (62.5 MHz, CDCl3) 169.2, 168.6, 76.2, 55.9, 51.5, 51.0, 46.8, 33.4, 28.3, 21.3;m/z (C.I) 216 (MH+, 9%), 159 (10%), 150 (4%), corresponding to C10H17O5, with a calculated mass of 216.0998 and a found mass of 216.0935. Further elution provided product (4) (18 mg, 26% yield), with the following spectral data: υmax (thin film/cm-1) 2957, 1742, 1733; δH (250 MHz, CDCl3) 5.06 (1H, t, J 5.3 Hz, CHO), 3.73 (3H, s, CO2CH3), 3.68 (1H, d, J 3.3 Hz, CHC=O), 3.14-3.10 (1H, m, CHCHC=O), 1.98-1.56 (6H, m, CH2); δC (62.5 MHz, CDCl3) 176.9, 168.5, 86.5, 54.8, 53.7, 43.5, 33.5, 32.9, 23.9;m/z (C.I) 185 (MH+, 45%), 141 (51%), 134 (43%), 124 (67%), 101 (77%), 84 (54%) corresponding to C9H13O4, with a calculated mass of 185.0814 and a found mass of 185.0806.4. Conclusions
- In conclusion, the successful alkylation of 2-chlorocyclopentanone with dimethyl malonate utilizing ionic liquids as reaction media underscores the notable efficiency and environmental potential of these innovative solvents. The significant enhancement in yield observed with [BMIM]BF₄ and [EMIM]OTf, in comparison to conventional dichloromethane (DCM), illustrates the distinctive solvation environment and polarity of ionic liquids, which promote enolate formation and stabilize critical reaction intermediates. In addition to their performance advantages, the low volatility and recyclability of ionic liquids provide substantial environmental benefits, aligning with the principles of green chemistry. This study not only demonstrates the feasibility of substituting hazardous organic solvents with ionic liquids but also reinforces their capacity as versatile platforms for advancing sustainable carbon–carbon bond-forming transformations. The findings contribute to the potential for broader adoption of ionic liquid-based methodologies in contemporary synthetic protocols.
ACKNOWLEDGEMENTS
- The authors wish to express their sincere appreciation to Professor Laurence M. Harwood for his initial conception of the idea.Nicholls State University, Thibodaux, Louisiana, USA, and the University of Reading, United Kingdom, provided the essential infrastructure and steadfast support that enabled the execution of this research.Author contributions: Bello Makama undertook the research, while Laurence M. Harwood provided initial supervision. Clinical Trial Number: Not applicable.Funding: This study did not receive any external funding.Data availability: The manuscript is not associated with any data.Declarations:Ethics approval and consent to participate: Not applicable.Consent to publication: Not applicable.Competing interests: The authors declare that there are no competing interests.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML