-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2025; 15(2): 50-58
doi:10.5923/j.chemistry.20251502.03
Received: Apr. 25, 2025; Accepted: May 19, 2025; Published: Jun. 13, 2025

Proximate Analysis and Physicochemical Properties of Oil from Four Avocado Cultivars from Murang’a County, Kenya
Samuel N. Wanjiru, Sylvia A. Opiyo, Peter W. Njoroge, Beatrice Mugendi
Department of Physical and Biological Sciences, Murang’a University of Technology, Kenya
Correspondence to: Samuel N. Wanjiru, Department of Physical and Biological Sciences, Murang’a University of Technology, Kenya.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Avocado has a high nutritional value with a high content of unsaturated fatty acids, vitamins, phytochemicals, fiber, protein, and minerals such as magnesium and potassium. However, it is difficult to determine the harvesting stage especially for green-skinned varieties. Maturity of avocado is determined by analysis of parameters such as maturity index, oil content and minerals content. However, several factors affect chemical the profile including seasonal variation, cultivar and geographical growing area. The objective of this study was to determine the proximate and physicochemical properties of avocado from Murang’a County, Kenya. Samples of four different cultivars were collected from three geographical zones. Proximate analysis was done on fresh avocado pulp. Oil was extracted from avocado pulp using petroleum ether. The proximate composition of avocado of the four cultivars ranged as follows: moisture content was 59.30-84.31%, ash content was 0.96-1.53%, dietary fibre content was 6.43-1.83% and oil content was 59.44-75.32%. The physicochemical properties of the oil samples ranged as follows: specific gravity 0.85-0.92; acid values 0.18-0.98 mg KOH/g; saponification value 123.42-187 mg KOH/g; iodine value 54-107g I2/100g; and peroxide values 0.9-2.3 Meq O2/kg. Altitude significantly influenced the proximate and physicochemical properties of avocado oil across different cultivars. Understanding these variations is crucial for optimizing avocado cultivation, processing, and utilization for nutritional and industrial applications.
Keywords: Avocado, Cultivar, Altitude, Proximate composition, Physicochemical properties
Cite this paper: Samuel N. Wanjiru, Sylvia A. Opiyo, Peter W. Njoroge, Beatrice Mugendi, Proximate Analysis and Physicochemical Properties of Oil from Four Avocado Cultivars from Murang’a County, Kenya, American Journal of Chemistry, Vol. 15 No. 2, 2025, pp. 50-58. doi: 10.5923/j.chemistry.20251502.03.
1. Introduction
- Since ancient times, plants have been an essential source of nourishment, providing vital nutrients that sustain human life while also serving as powerful tools for healthcare and disease treatment [1-12]. Beyond their role in food, plants contain a wealth of secondary metabolites-bioactive compounds that exhibit diverse pharmaceutical applications [13-21]. Many of these natural substances have paved the way for groundbreaking drug discoveries, offering potential leads for the development of new medications to combat infections and other ailments [22-26]. As science continues to explore the vast biochemical arsenal found in nature, plants remain invaluable in shaping both nutrition and modern medicine [27-38]. Persea americana Mill., commonly known as avocado, is a tropical evergreen fruit belonging to the Lauraceae family. It is classified as a climacteric fruit, meaning it continues to ripen after harvesting due to ethylene production. The fruit has generated great interest in recent years as a natural functional food due to its high nutritional value and health benefits. Avocado has a high nutritional value with a high content of unsaturated fatty acids, vitamins, phytochemicals, fiber, protein, and minerals such as magnesium and potassium [39,40]. Avocado fruits should be allowed to maintain sufficient maturity to be palatable upon ripening [41,42]. The question of when to start harvesting avocado fruit is of great commercial importance. It is difficult to determine the harvesting period especially for green-skinned varieties [43,44]. Maturity of avocado in several countries is determined by chemical analysis. Several researchers have studied different components of avocado pulp in order to determine their quality. Some of the components studied include maturity index, oil content, digestibility coefficient and amount of minerals in the pulp. These components have variations from season to season for each cultivar, geographical growing area, maturity, soil conditions and farm management practices. Moisture or dry matter content determination has been the most common indicator used for avocado fruit maturity. However, other complementary indices can be considered, such as flesh softening and change of skin colour from green to black in some cultivars such as Hass. Dry matter is dependent on variety, region and season. This is a more accurate method of determining the maturity of avocados. 24% and 30% dry matter are the minimum specification for fresh fruits maturity and for oil processing respectively in Kenya. Dry matter increases as the fruits mature [45-47]. Percentage oil content is another common indicator used for avocado fruit maturity [48]. Starting from 1925, a minimum standard of 8% oil content in the pulp of avocado fruits was used in the California avocado industry in the United States but since the eighties, they began using minimum oil content percentages for each cultivar: 10.0% for Fuerte and 11.2% for Hass [41,47,49]. The amount of oil depends on cultivar, maturity, geographical growing area, ripening stage and extraction method [50-52]. In a study of fatty content of three cultivars namely Hass, Breda and Margarida, there was significant difference in the percentage oil content between cultivars and different ripening stages [45]. In another study, fatty acid composition of oils from four avocado cultivars namely Quintal, Fortuna, Margarida and Hass similar observations were made [53]. Avocado harvested in early, mid and late season yielded significantly different percentage oil content [54].Dietary fibre (DF) is found in wholegrain cereals, fruit and vegetables [55]. Fibre is made up of the indigestible parts or compounds of plants, which pass relatively unchanged through the stomach and intestines. Fibre is mainly a carbohydrate. The main role of fibre is to keep the digestive system healthy. Diets, deficient in DF, lead to a number of diseases such as constipation, hiatus hernia, appendicitis, diabetes, obesity, coronary heart diseases and gallstones [56]. Consumption of adequate amounts of DF reduces the risk of above-mentioned diseases [57]. Studies have shown that avocado oil has a high digestibility coefficient greater than 93.8%. Dietary fibre content varies with cultivar and the part of the fruit analyzed and geographical growing area [41,58].Physicochemical properties of an oil are an important variable in considering its applications since physicochemical properties influence oil quality. Physicochemical properties like acid value, iodine value, saponification value, peroxide value provide information in determining the suitability of oil for consumption [59]. Further, when designing technological processes physicochemical properties of oil are important parameters to manufacturers [60].Oils contain various triglycerides in different proportions. Specific gravity and viscosity of oil depend on the type of triglycerides present [53,61]. Specific gravity and viscosity decrease with increase in saturation and increases with unsaturation and polymerization [50]. Refractive index of an oil is the ratio of speed of light at a defined wavelength to its speed in the oil/fat itself. This value varies with wavelength and temperature, the degree and type of unsaturation, the type of substitutions of component fatty acids and with accompanying substances. Refractive index is widely used in quality control to check for the purity of materials and to follow hydrogenation and isomerization of an oil [62]. Acid value of an oil indicates the amount of free fatty acid present that affect its stability and quality. It is measured by milligrams of KOH required to neutralize the fatty acids in one gram of lipid. Acid value is one metric used to assess an oil's edibility and suitability for industrial uses like paints is its acid value [63]. An oil with a low acid value is stable for a long time and is protected from peroxidation and rancidity. The avocado pulp's natural antioxidants, including vitamins A and C, as well as other potential phytochemicals, like flavonoids, may be responsible for the stability [64]. Although an oil with a high acid value may not be edible for use in cooking, it can be helpful for making paints, liquid soap, and shampoos [65]. Additionally, oils with a noticeable acidity level are a sign that the plant may be toxic to cattle [66].The saponification number or saponification value is an important parameter used for the characterization and assessment of the quality of edible fats and oils. It is measured by the number of milligrams of NaOH/KOH needed to hydrolyse one gram of fat. The average molecular weight of the triacylglycerols in a sample is indicated by the saponification number. Lower molecular weight fatty acids are present when the saponification value is high. This indicates that the quality of the oil is good for making cooking salads [66]. The oil may not be edible and should only be used to make soap, oil-based ice cream, and shampoos if it has a low saponification value, which indicates that it contains a high proportion of higher fatty acids [67,68]. The process of saponification involves treating a neutral fat with an alkali to break it down into glycerol and fatty acids (Figure 1).
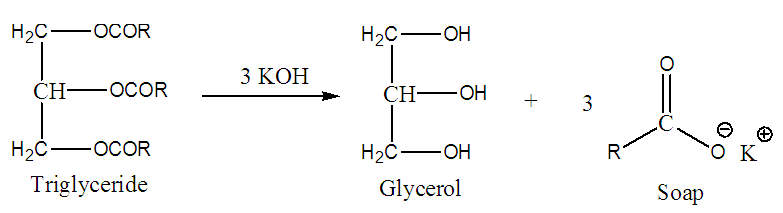 | Figure 1. Saponification of natural fat |
2. Materials and Methods
- Sampling and sample preparationMature avocado fruits were picked from different farms selected randomly and purposively from the three different climatic zones In Murang’a County. The farms from the upper zone were distributed above 2200m above sea level (asl) in Kanderendu area, Kangari Ward in Kigumo constituency on coordinates (0°47’S, 36°49’E). The farms from the middle zone were distributed between 1700-2200m asl in Gatanga and Kihumbu-ini wards in Gatanga constituency on coordinates (0°55’S, 36°57’E). The farms from the lower zone were distributed between 1300-1550m asl in Ichagaki and Kamahuha areas, Maragua constituency on coordinates (0° 47’S, 36º49’E). The samples were identified and labelled accordingly. The samples were sorted and kept for 4-7 days to ripen. The ripe fruits were thoroughly washed with water to remove mud and any other materials on its surface. The fresh fruit were weighed then cut longitudinally to remove the seed. The peel was then removed and the pulp cut into small pieces and crushed in a mortar.Lipids ExtractionThe extraction of oil was done in a Soxhlet extractor using petroleum ether using the method described by Manaf et al [73]. A homogenous sample was dried in an oven for 24 hours at 70°C then ground into fine powder. 10g of the dried sample was mixed with petroleum ether. The mixture was transferred into a Soxhlet extractor for 6-8 hours at 40-60°C. The extract was then evaporated in a rotary evaporator at 95°C to evaporate the solvent. The lipid was weighed and expressed in % w/w on the dried fruit weight basis.Moisture ContentMoisture content was determined by the method described by Carvalho et al 45 as follows; Two grams of the sample was accurately weighed in clean dry crucible (W1). The crucible was oven dried at 100 -105°C until a constant weight was obtained. The crucible was then placed in the desiccator for 30 min to cool. After cooling, it was weighed again (W2) .The percent moisture was calculated using the formula below:% Moisture = (W1-W2)/W0 × 100Where W0 is Weight of sample (g), W1 is Initial weight of crucible + Sample (g), W2 is Final weight of crucible + Sample (g).Ash ContentAn empty crucible was weighed and the weight recorded as (W0). Two grams of the sample was placed in the crucible and the weight recorded as (W1). The crucible was placed in a furnace at 550°C for 3 hours. The crucible was allowed to cool and weight recorded as (W2). The percentage of the ash content was calculated using the formula below;% Ash content = (W2-W0)/(W1-W0)× 100Where W0 is weight of empty crucible (g), W1 is weight of crucible + powdered sample (g), W2 is weight of crucible + ash sample (g).Dietary FiberCrude fibre was determined by the method described by Maitera et al 2014 as follows: 5g sample was digested into trichloroacetic acid for 40 min. The residue was filtered and washed with boiling water and acetone. The residue was heated at 105°C in an oven. The residue was scrapped and weighed (W1), then ashed in a furnace at 550°C for 2 hours. The sample was then cooled and weighed (W2). The fibre content was calculated using the formula below.%Fibre = (W1-W2)/W0*100Where W0= weight of sample, W1= weight of dried sample, W2= weight of ash sample.Determination of Physicochemical Properties of OilThe analyses of the physicochemical properties (specific gravity, acid value, saponification value, iodine value and peroxide value) of avocado oil was done as described by the AOAC methods [74].Specific GravityA clean and dry pycnometer was weighed and its weight recorded as x. The pycnometer was then filled with water, the temperature was adjusted to 25°C in a water bath and weighed again. The weight was recorded as y. The pycnometer was emptied and dried. The pycnometer was then filled with oil and weighed again. The weight recorded as z. The formula below was used to calculate the Specific gravity [74].Specific gravity= z-x/ y-xwhere x is mass of empty pycnometer, mass, y is mass of pycnometer filled with distilled water, z is mass of pycnometer filled with oil.Acid ValueAcid value was determined by the method described by Nasri et al. [52] as follows; Two grams of oil were dissolved into solvent mixture of 25 ml of ethanol 99% and 25 ml of diethyl-ether, two drops of phenolphthalein added and the solution titrated with 0.5N potassium hydroxide until a pink end-point was reached. The acid value was determined by the formulaAcid value= 56.1V*N / W (mg of KOH/g of oil)where V= number of ml of the potassium hydroxide used, N =Normality of potassium hydroxide used and W = mass of oil used.Saponification value The saponification value was determined by taking 1.0 g of oil sample in a conical flask to which 15 ml 1 N KOH (prepared by dissolving 56.0 g of KOH pellets in 400cm3 of water and diluting it to 1000ml in a clean volumetric flask) is added and 10 ml of distilled water and heated under a reserved condenser for 30–40 minutes to ensure that the sample fully dissolves. After that, sample was cooled, phenolphthalein added and titrated with 0.5 M of HCl until a faint pink persisted for 15 seconds. 25 ml of KOH in which no oil is added was titrated with the standard HCl to determine the alkali originally added [74].Saponification value = (VB-VW) *56.1N/W (mg KOH/g of oil)Where VB is the volume of the Blank, VW is volume of the sample N=1.Iodine Value In order to determine the iodine value, 0.01N sodium thiosulphate was prepared by dissolving 25 g of sodium thiosulphate in freshly boiled water and the solution made up to 1000ml in a volumetric flask. The solution was then standardized using 0.01N potassium dichromate solution [74]. The normality of the sodium thiosulphate was then determined by the formula;F = 20 x F’/Vs-VbWhere F is the normality of sodium thiosulphate, F’= 0.01, Vs is the volume of potassium dichromate used for the sample and Vb is the volume of the blank.The 10% potassium iodide was prepared by dissolving 10g of KI in 100ml of starch solution prepared by dissolving 1.5g of starch in water and boiling it for 30 minutes.Iodine value was determine by the method described by Nasri et al. [52] as follows: 0.4g of the oil sample was accurately weighed into a conical flask. 20ml of CCl4 was added followed by 25cm3 of wijs solution, then shaken vigorously for 30 seconds. The mixture was stored in the dark for 2.5 hours. A mixture of 20ml of 10% KI with 125ml of water was added. The solution was then titrated with 0.01N Na2S2O3 using starch indicator until the blue black coloration changed to colourless. Blank determination was done without oil.IV= (B-S) *N*12.69 /W (g of iodine/100 g of oil)Where B is the quantity of sodium thiosulphate used for blank, S is the quantity of thiosulphate for sample, N is the normality of thiosulphate solution, W is the weight of the oil sample. Peroxide value (PV) Peroxide value is a measure of peroxides contained in the oil. PV was determined by measuring iodine released from potassium iodide. A 0.3 g of oil sample was dissolved in a 10ml of a solvent made by mixing glacial acetic acid and chloroform in the ratio 3:2. 1ml of saturated KI (prepared by dissolving 100 g of KI in 70ml of distilled water) was added to the sample and the mixture was immediately stoppered and store in the dark for 5 minutes. 20ml of distilled water was then added and the amount of iodine liberated from KI by the oxidative action of peroxides present in the oil was determined by titration with 0.01N sodium thiosulphate using starch solution as an indicator [74]. PV (meq of O2/kg of oil) = (Vs-Vb) x F x N x 1000 /WWhere Vb is the volume of sodium thiosulphate used for blank, W is the weight of sample, Vs is the volume of sodium thiosulphate consumed by the sample oil, N is the normality of standard sodium thiosulphate and F is the factor of 0.01N sodium thiosulphate.
3. Results and Discussion
- Proximate CompositionFuerte grown in the zone 3 had the lowest moisture content of 59.30% while Pinkerton grown in zone 1 had the highest moisture content of 84.32%. The moisture content was significantly different between cultivar and geographical growing environment (Figure 2). The difference in the moisture content may be due to difficulties in determining the maturity of the fruits from the field. However this does not mean the fruits grown in the same area could not attain the same maturity level [45]. According to Agriculture and Food Authority, Horticultural Crops Directorate Hass and Fuerte avocados harvesting guide, fruits should attain at least 20% dry matter content and not less than 8% oil content. In this line all the fruits collected from the farms met this criteria except Pinkerton grown in zone 1 with a dry matter content of about 16%. From these results, avocado fruits contain high moisture content and therefore they have shorter shelf-life. The moisture content values are similar to those reported from other studies [49].
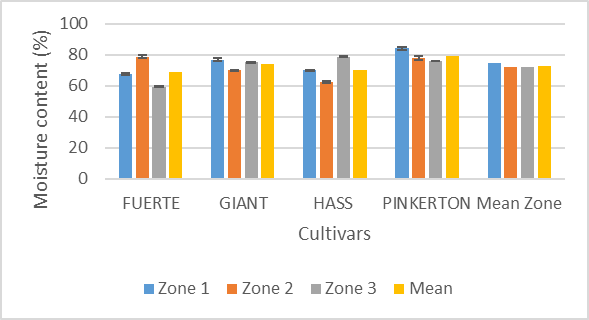 | Figure 2. Variation of moisture content with cultivar and zone |
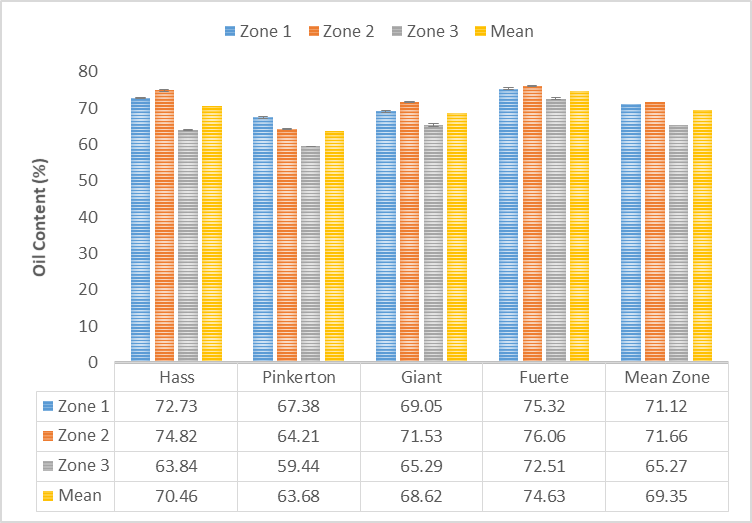 | Figure 3. Effect cultivar and zone on oil content of avocado pulp |
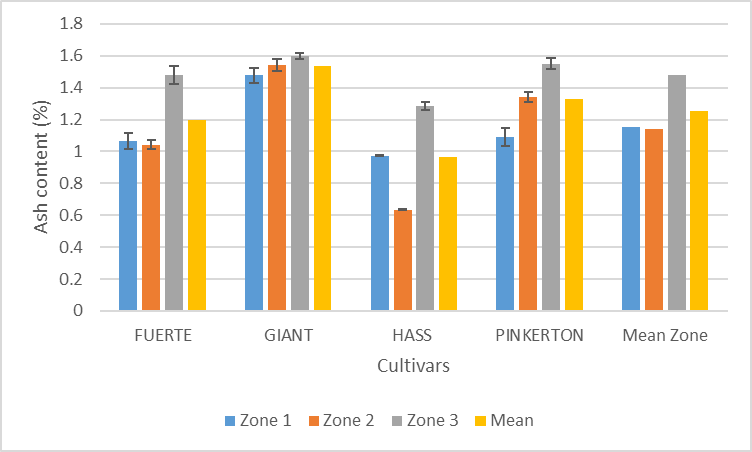 | Figure 4. Effect cultivar and zone on ash content of avocado pulp |
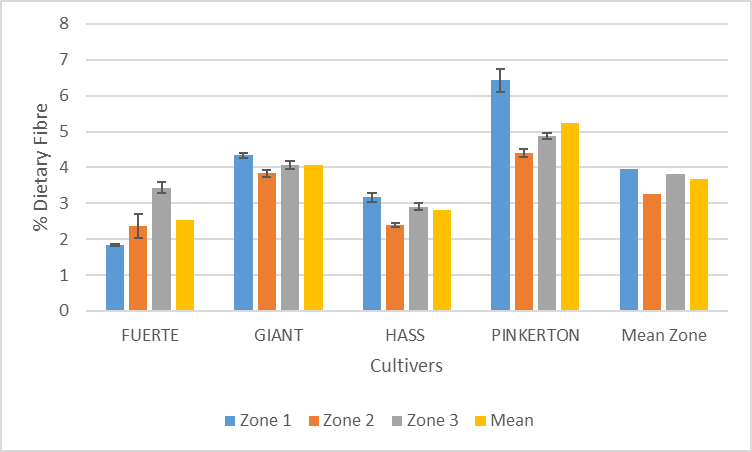 | Figure 5. Variation of dietary fibre content of avocado pulp with cultivar and zone |
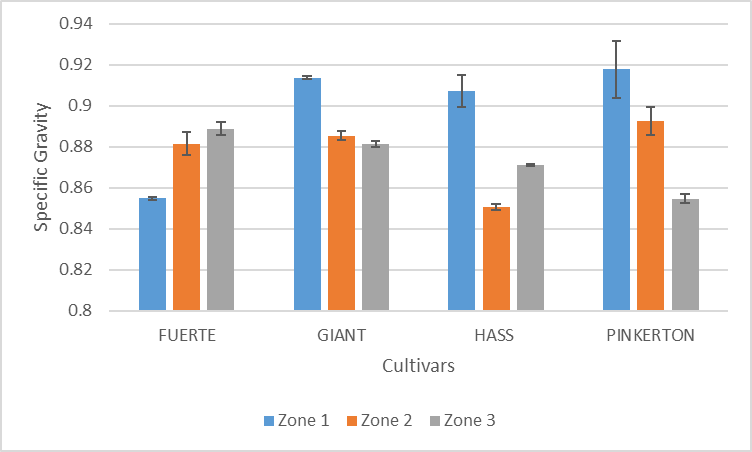 | Figure 6. Variation of specific gravity of avocado oil with cultivar and zone |
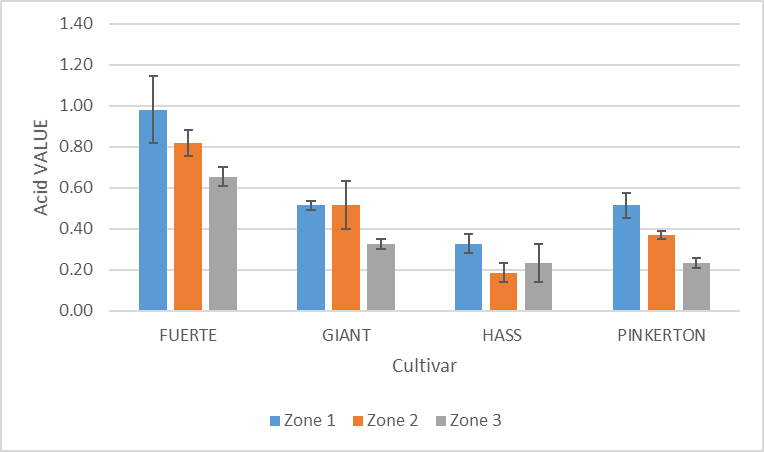 | Figure 7. Variation of acid value of avocado oil with cultivar and zone |
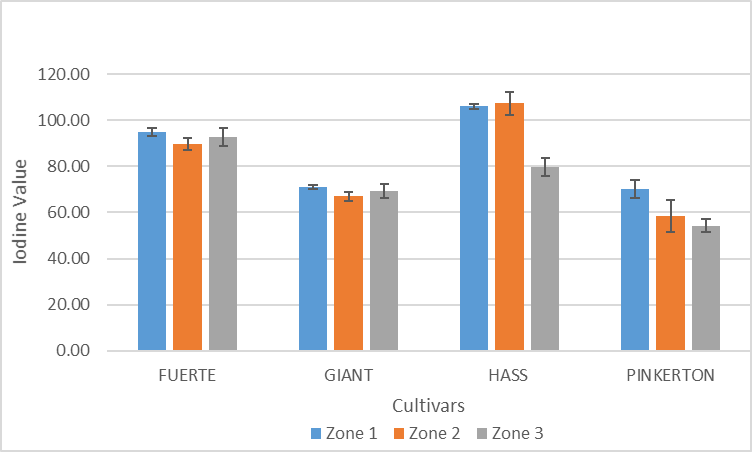 | Figure 8. Variation of iodine value of avocado oil with cultivar and zone |
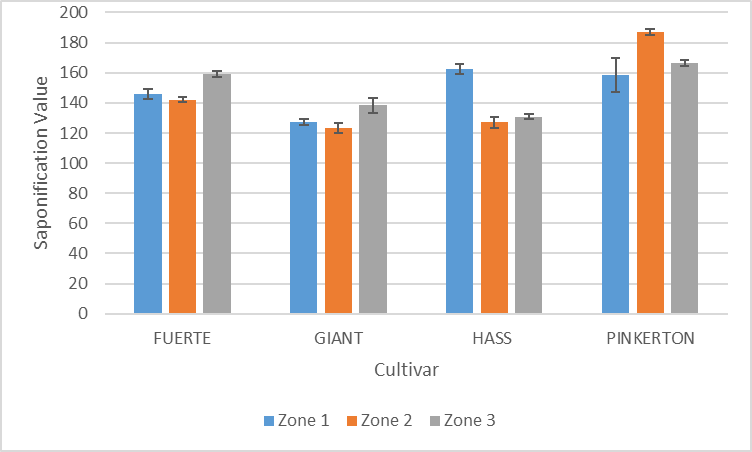 | Figure 9. Variation of saponification value of avocado oil with cultivar and zone |
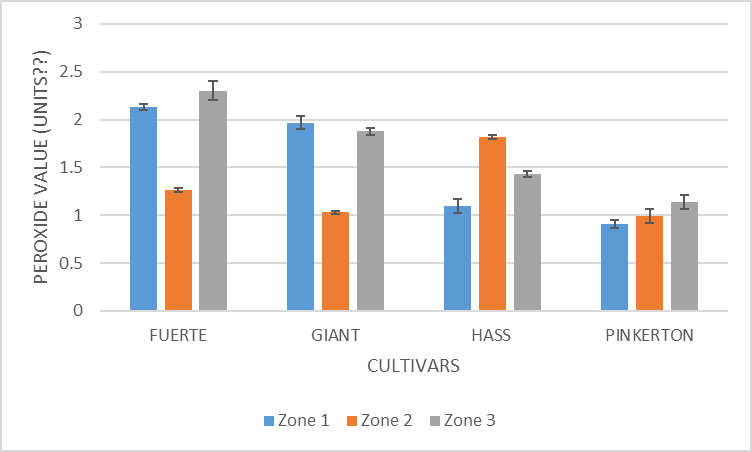 | Figure 10. Peroxide value of oils from different cultivars and zones |
4. Conclusions
- This study aimed to investigate the influence of the cultivar and altitude on the proximate properties including the moisture, ash, oil and dietary fibre contents of avocado cultivars grown in Murang’a County. The physicochemical properties of oil from the avocado samples including specific gravity, acid value, saponification value, iodine value and peroxide values were also investigated. This study highlights the influence of altitude on the proximate composition of avocado pulp and the physicochemical properties of avocado oil across different cultivars. The findings suggest that altitude plays a significant role in determining nutrient content, oil yield, and quality parameters. Understanding these variations is crucial for optimizing avocado cultivation, processing, and utilization for nutritional and industrial applications. Further research could explore the mechanisms behind these differences, helping to enhance avocado-derived products for both local and global markets.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML