-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2025; 15(1): 20-24
doi:10.5923/j.chemistry.20251501.03
Received: Mar. 16, 2025; Accepted: Apr. 10, 2025; Published: Apr. 16, 2025

Influence of Extraction Technique on Phenol and Flavonoid Content in Terminalia glaucescens: Ultrasound versus Maceration
Lanciné Traoré1, 2, Marie Rosine Atsain-Allangba2, Delphine Ebalah Monyn-Kouamé3, Janat Akhanovna Mamyrbekova-Békro2, Yves-Alain Békro2
1UFR-Sciences et Technologies, Université de Man, BP 20 Man, Côte d’Ivoire
2Laboratoire de Chimie Bio Organique et de Substances Naturelles, UFR-SFA, Université Nangui Abrogoua, 02 BP 801 Abidjan 02, Abidjan, Côte d’Ivoire
3UFR-Ingénierie Agronomique Forestière et Environnementale, Université de Man, BP 20 Man, Man, Côte d’Ivoire
Correspondence to: Lanciné Traoré, UFR-Sciences et Technologies, Université de Man, BP 20 Man, Côte d’Ivoire.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

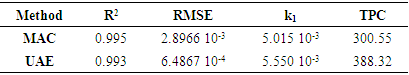
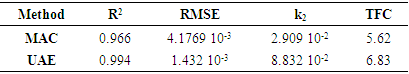
The extraction of bioactive compounds from plant matrices remains a critical focus in pharmacological, nutraceutical, and food applications. This study evaluates the efficiency of ultrasound-assisted extraction (UAE) and conventional maceration (MAC) for recovering phenols and flavonoids from Terminalia glaucescens. A second-order kinetic model was applied to characterize the extraction kinetics and compare the performance of both methods. Quantitative analyses revealed significant differences in yield and kinetics: UAE demonstrated accelerated extraction rates and superior overall yields compared to MAC. Specifically, UAE achieved higher phenol (385.5 ± 2.1 µg GAE/mg DM vs. 308.7 ± 1.9 µg GAE/mg DM) and flavonoid (6.3 ± 1.1 µg QE/mg DM vs. 4.5 ± 1.3 µg QE/mg DM) contents, alongside faster kinetic constants (₂ = 5.550 × 10⁻³ mg DM/µg GAE·min⁻¹ vs. 5.015 × 10⁻³ mg DM/µg GAE·min⁻¹ for polyphenols; k₂ = 8.832 × 10⁻² mg DM/µg QE·min⁻¹ vs. 2.909 × 10⁻² mg DM/µg QE·min⁻¹ for flavonoids). These results highlight the efficacy of UAE in enhancing bioactive compound recovery, supporting its potential for industrial-scale optimization of phytochemical extraction processes.
Keywords: Terminalia glaucescens, Bioactive compounds, Ultrasounds-assisted extraction, Polyphenols, Flavonoids
Cite this paper: Lanciné Traoré, Marie Rosine Atsain-Allangba, Delphine Ebalah Monyn-Kouamé, Janat Akhanovna Mamyrbekova-Békro, Yves-Alain Békro, Influence of Extraction Technique on Phenol and Flavonoid Content in Terminalia glaucescens: Ultrasound versus Maceration, American Journal of Chemistry, Vol. 15 No. 1, 2025, pp. 20-24. doi: 10.5923/j.chemistry.20251501.03.
Article Outline
1. Introduction
- Terminalia glaucescens (Combretaceae), a plant widely utilized in traditional medicine for treating sores, nervous disorders, inflammatory conditions, and microbial infections, has emerged as a promising source of bioactive compounds, particularly polyphenols and flavonoids [1-3]. These secondary metabolites, integral to plant defense mechanisms, exhibit significant antioxidant, anti-inflammatory, and antimicrobial properties, positioning them as key targets for pharmacological exploration and therapeutic development [4,5]. Phenol compounds, renowned for their ability to scavenge reactive oxygen species (ROS) and alleviate oxidative stress [6], have driven innovations in extraction methodologies to optimize yield and bioactivity [7]. The choice of extraction technique critically impacts not only the quantity of recovered compounds but also their stability, purity, and functional efficacy [8]. While conventional maceration—a cost-effective method involving prolonged solvent immersion at ambient temperatures—remains widely used for phytochemical extraction [9], its limitations include extended processing times and potential degradation of heat-sensitive compounds [10]. In contrast, ultrasound-assisted extraction (UAE) leverages acoustic cavitation to enhance mass transfer, significantly reducing extraction duration while improving efficiency [11]. Recent studies have highlighted the need for systematic comparisons of UAE and maceration in recovering total phenolic content (TPC) and flavonoids from plant matrices [12-14]. This study quantitatively evaluates the efficacy of UAE and maceration in extracting total phenolics and flavonoids from Terminalia glaucescens, providing critical insights for optimizing phytochemical recovery in industrial and pharmacological applications.
2. Materials and Methods
2.1. Plant Materials and Reagents
- Roots of Terminalia glaucescens were collected in Kani, northern Côte d'Ivoire, and authenticated at the National Floristic Center. The plant material was thoroughly washed with deionized water, air-dried at room temperature for 14 days, and then ground into a fine powder using a mortar and pestle. Analytical-grade solvents such as methanol, ethanol, and acetone were used throughout the extraction procedures. The ultrapure water was prepared in the laboratory. Folin–Ciocalteu reagent, aluminum chloride, sodium carbonate, and gallic acid were used to determine total polyphenolic content, while quercetin served as the standard for flavonoid assays. All chemicals and reagents were obtained from Sigma-Aldrich (Saint Louis, USA) and used without further purification.
2.2. Extraction Procedures
- Two extraction methods were employed: ultrasound-assisted extraction and conventional maceration.
2.2.1. Ultrasound-Assisted Extraction (UAE)
- 20 mg of powdered plant material is used. The choice of plant material will influence the compounds extracted, so it’s important to know the plant species and its properties. A solvent mixture of 60% acetone in ultrapure water is prepared. Acetone is a polar solvent and is effective for extracting a variety of phytochemicals, including flavonoids, phenolics, and some alkaloids. The water component helps to solubilize more polar compounds. The mixture is subjected to sonication at a frequency of 40 kHz and a power of 240 kW. This high-power level suggests enough energy is being applied to facilitate the extraction process, enhancing the penetration of the solvent into the plant material and promoting the release of the desired compounds. The temperature is maintained at 40°C, which can help in dissolving more hydrophilic compounds while preventing thermal degradation of sensitive phytochemicals. Samples are taken every 5 minutes to monitor the extraction process over time. This time-course analysis could help identify the optimal extraction time for maximum yield of the target compounds. After each sampling, the mixture is centrifuged at 3000 rpm to separate the solid plant material from the liquid supernatant. The supernatant contains the extracted compounds. The supernatant is then analyzed.
2.2.2. Conventional Maceration (MAC)
- 20 mg of powdered plant material is weighed and placed in an Erlenmeyer flask. 12 mL of a 60% acetone solution is prepared by mixing acetone with ultrapure water. The mixture is heated to 40°C, which helps to facilitate the extraction of compounds from the plant material. Continuous gentle agitation is maintained for 40 minutes to enhance the extraction efficiency. Every 5 minutes, a sample of the extract is taken for analysis or further processing, similar to methods used in ultrasound-assisted extraction (UAE).
2.3. Determination of Total phenol Content (TPC)
- The total polyphenol content was measured by the Folin-Ciocalteu method (15). 1 mL of an aliquot of each extract was taken for analysis. 500 µL of Folin-Ciocalteu reagent (diluted 1/10 with ultrapure water) was added to the sample. This reagent acts as an oxidizing agent that reacts with phenolic compounds, resulting in a color change. After a 5-minute incubation period, 500 µL of saturated sodium carbonate solution was added. This step helps to neutralize the acidity and allows for the formation of a blue-colored complex that can be measured spectrophotometrically. The mixture was incubated at room temperature in the dark for 40 minutes to allow the reaction to complete and stabilize. The absorbance of the resultant solution was measured at a wavelength of 765 nm using a UV-Visible spectrophotometer. This wavelength corresponds to the peak absorbance of the blue complex formed in the reaction. The TPC was expressed as micrograms of gallic acid equivalents per milligram of dry matter (µg GAE/mg DM). Gallic acid is commonly used as a standard reference for phenol quantification due to its well-characterized chemistry and reactivity with the Folin-Ciocalteu reagent.
2.4. Determination of Total Flavonoid Content (TFC)
- TFC was determined by the aluminum chloride colorimetric method (16). A 2 mL aliquot of the extract was mixed with 1 mL of 5% aluminum chloride solution. The mixture was thoroughly stirred and after 30 min the absorbance was read at 434 nm. The flavonoid content was quantified using a quercetin calibration curve, and the results were expressed as micrograms of quercetin equivalents per milligram of dry matter (µg QE/mg DM).
2.5. Kinetic Modeling and Data Analysis
- The extraction kinetics were modeled using a second order kinetic equation. The model was represented by:
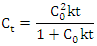 where Ct is the extract concentration at time t, C0 is the equilibrium concentration, and k is the second order extraction rate constant. Non-linear regression analysis was applied to fit the kinetic data. The reliability of the fit was evaluated through the coefficient of determination (R²), and kinetic parameters were compared by analysis of variance (ANOVA). P-values were calculated with a significance level of 0.05.Data were processed using statistical software (RStudio 2024.12.1 Build 563). Each extraction experiment was performed in triplicate, and the results are presented as mean ± standard deviation.
where Ct is the extract concentration at time t, C0 is the equilibrium concentration, and k is the second order extraction rate constant. Non-linear regression analysis was applied to fit the kinetic data. The reliability of the fit was evaluated through the coefficient of determination (R²), and kinetic parameters were compared by analysis of variance (ANOVA). P-values were calculated with a significance level of 0.05.Data were processed using statistical software (RStudio 2024.12.1 Build 563). Each extraction experiment was performed in triplicate, and the results are presented as mean ± standard deviation.3. Results and Discussions
3.1. Extraction Yields
- The yield of phenols and flavonoids varied markedly with the extraction method and time. Figures 1 and 2 illustrate the cumulative yield over time for both UAE and MAC. Ultrasound-assisted extraction notably enhanced the recovery of bioactive compounds. At 40 minutes, UAE reached a total phenol content of 385.5 ± 2.1 µg GAE/mg DM compared to 308.7 ± 1.9 µg GAE/mg DM observed for maceration. Similar trends were observed for flavonoid content, where UAE yielded 6.3 ± 1.1 µg QE/mg DM in contrast to 4.5 ± 1.3 µg QE/mg DM via maceration.
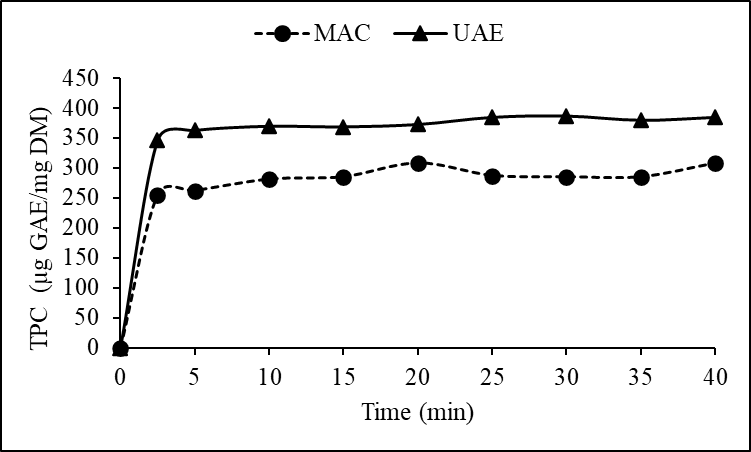 | Figure 1. Comparison of phenol extraction yields (TPC) between ultrasound (UAE) and maceration (MAC) |
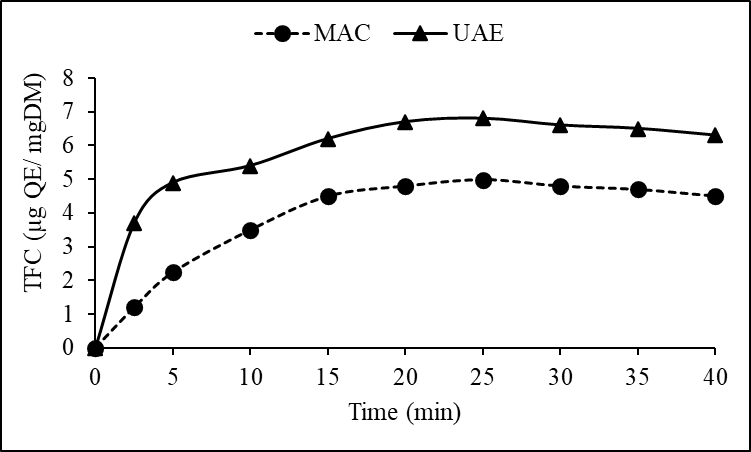 | Figure 2. Comparison of flavonoid extraction yields (TFC) between ultrasound (UAE) and maceration (MAC) |
3.2. Kinetic Model Fitting
- The kinetics of extraction were analyzed using the second order model. Data fitting yielded high R² values (≥ 0.99) and small RMSE values (≤ 4.18 10-3) for both UAE and MAC, indicating excellent conformity with the proposed model. The extraction process can be depicted by the rearranged kinetic model equation:
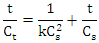 This formulation assisted in linearizing the data (Figures 3 and 4) for straight forward estimation of kinetic parameters through regression analysis.
This formulation assisted in linearizing the data (Figures 3 and 4) for straight forward estimation of kinetic parameters through regression analysis.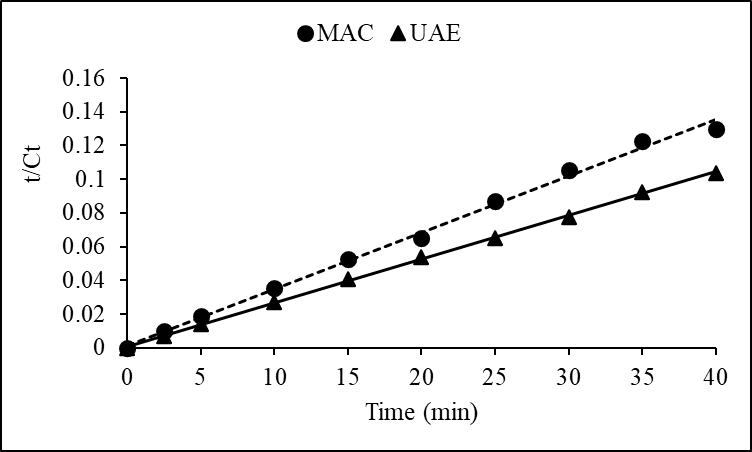 | Figure 3. Linearized form of the second-order kinetic model for polyphenol extraction |
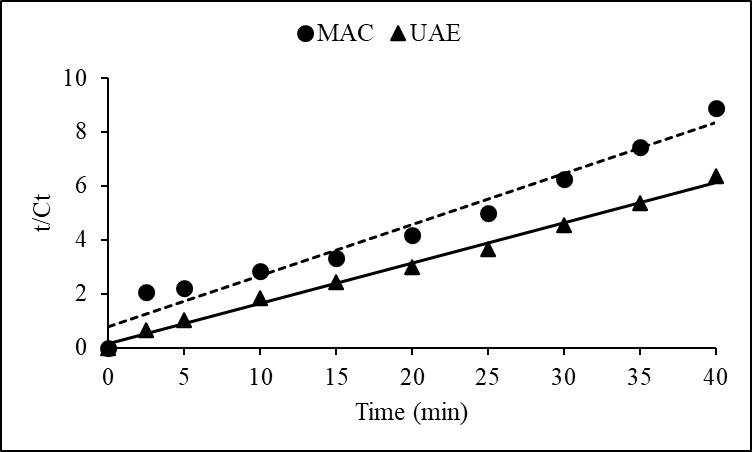 | Figure 4. Linearized form of the second-order kinetic model for flavonoid extraction |
|
|
4. Conclusions
- The study highlights the advantages of ultrasound-assisted extraction (UAE) over conventional maceration extraction (MAC) for extracting phenols and flavonoids from Terminalia glaucescens. The research utilized a second-order kinetic model to quantitatively characterize the extraction process, demonstrating that UAE yields higher rate constants and equilibrium yields compared to MAC. Statistical analysis supported these findings, confirming UAE's superior extraction efficiency and reduced processing time. The rigorous methodology and reproducibility of the experimental procedures lay a strong foundation for future research aimed at industrial applications. The enhanced extraction efficiency of UAE suggests it could be a viable alternative to traditional methods, particularly in the production of high-quality nutraceuticals and herbal products. Future research directions should focus on understanding the mechanisms behind ultrasound-induced cavitation and its effects on the stability of extracted compounds. Additionally, assessing the scalability of UAE, including its energy requirements and cost-effectiveness, will be critical for its implementation in commercial extraction processes.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML