-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2025; 15(1): 10-19
doi:10.5923/j.chemistry.20251501.02
Received: Feb. 17, 2025; Accepted: Mar. 22, 2025; Published: Mar. 28, 2025

Removal of Nitrites from Wetland Waters Based on Diazonium Silica
Aquiline Kathambi1, Peter W. Njoroge1, Sylvia A. Opiyo1, Isaac Waweru2, Benson M. Mwangi1
1Department of Physical and Biological Sciences, Murang’a University of Technology, Kenya
2Department of Chemistry, Kenyatta University, Kenya
Correspondence to: Peter W. Njoroge, Department of Physical and Biological Sciences, Murang’a University of Technology, Kenya.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Diazonuim silica was prepared and used to remove nitrites in wetland waters. Raw silica was chlorinated using phosphorous pentachloride (PCl5). Chlorinated silica was further reacted with ethylenediamine (EDA) under reflux to give aminated silica. Thereafter, aminated silica was protonated to yield diazonium silica which was used as the adsorbent. The prepared diazonium silica material was characterized using Fourier Transform Infrared (FT-IR) showing bands at wavenumbers 1049 and 791 cm-1 of the anti-symmetric and symmetric stretching modes (Si-O-Si) of SiO4. The shift in spectra band of Si-O-Si from 1051cm-1 in raw silica to 1049cm-1, was attributed to diazonium silica. Additionally, a new open chain azo (N=N) group band was found in the region 1575cm-1 -1630cm-1. The physical adsorption parameters examined using batch adsorption system including: pH, initial concentration, contact time and temperature were made using sorption models, adsorption kinetics and thermodynamics. The results obtained showed 3.0 as the optimal pH for adsorption of NO2-, 303K as the optimal temperature, optimum contact time as 60 minutes and optimal initial concentration as 20ppm. The adsorption capacity of nitrites using diazonium silica adsorbent was achieved at 16.45mg/g. For sorption of nitrites in diazonuim silica, Langmuir isotherm model was obeyed according to correlation coefficient R2 = 0.97182 close to unity. Adsorption kinetics of nitrites in diazonium silica pseudo second order was best obeyed while, thermodynamic parameters revealed that the sorption of nitrites into diazonium silica was exothermic in nature. From the results obtained, diazonium silica is an efficient adsorbent for adsorption of nitrites compared to raw silica.
Keywords: Sorption, Diazonium silica, Nitrites, Adsorption capacity, Kinetics thermodynamics
Cite this paper: Aquiline Kathambi, Peter W. Njoroge, Sylvia A. Opiyo, Isaac Waweru, Benson M. Mwangi, Removal of Nitrites from Wetland Waters Based on Diazonium Silica, American Journal of Chemistry, Vol. 15 No. 1, 2025, pp. 10-19. doi: 10.5923/j.chemistry.20251501.02.
Article Outline
1. Introduction
- Fresh water occupies about 2% of the earth surface. Water bodies are mainly targeted by industries by discharging industrial effluents to water bodies leading to pollution. Waste water is harmful not only to aquatic life but also highly harmful to human life. [1] Pollution in the environment and more importantly water, should be handled in a special way as it is a serious matter affecting many organisms at every level [2]. The most valuable and productive ecosystem in the world is the wetlands. The earth surface covered by the wetlands is 15%. Wetlands offer 40% ecosystem services is in the world. These services include habiting aquatic animals and plants, cultural identity, opportunity for tourism among others. Improving water quality, reducing food wastage, and managing anthropogenic release of greenhouse gases are among three regulating services with global significant. Wetlands are capable of trapping pollutants and nutrients in their soils. Trapping of nutrients by the wetlands leads to water purification [3]. Nitrogen and phosphorous are key compounds of fertilizers and considered as a limiting nutrients when they are not substituted. With increasing world’s population, growth of annual demand for nitrogen and phosphorous is expected to increase according to the projection made by FAO. The long term consumption of nitrogen and phosphate worldwide is estimated to reach 199.3 million tones that is 27.8% of phosphorous and 72.2% nitrogen in 2030 [4].Nitrite ions are termed as wide spread contaminants established in aqueous environment. Nitrite ions are a significant indicator of the quality of natural waters. Increase in the levels of nitrite ions in ground or surface waters is caused by agricultural activities like use of fertilizers. Another source of nitrite ions is from industrial effluents discharged in water bodies. Nitrite ions have adverse effects on human and some species of fish therefore its removal from water has received great attention in the recent years. Nitrite ions enter in the bloodstream of fish through the gills. In the bloodstream nitrite ions oxidizes iron in the haemoglobin resulting to a product known as methemoglobin causing respiratory distress due to loss in oxygen-carrying capacity in the blood [5]. A reaction between nitrite and secondary or tertiary amines may lead to the formation of mutagenic, carcinogenic and N-nitroso compounds ( nitrosoamines) which may lead to cancer of alimentary canal. WHO has set the maximum acceptable concentration of NO3- to be 50mg/L and for NO2- to be 3mg/L [6].Currently, methods used to reduce the levels of pollutants from water include adsorption, liquid-liquid extraction and precipitation. Adsorption method has been used for long a time as a way of purification and separation process in industries. Adsorption method has advantages such as operation simplicity, high efficiency and economy ease [7]. Studies utilizing adsorption techniques have been conducted with a significant interest in lowering the amounts of contaminants in waste waters and aquatic environments [8]. Various materials have been used to prepare adsorbents which are used to purify water. Adsorbents commonly used incudes activated carbon, zeolite, chitosan, industrial waste and clay minerals among others. Activated carbon has shown excellent ability to remove organic compounds such as phenolics, pharmaceuticals, dyestuffs, pesticides and metal ions due to its high porosity nature [9]. Although the application of activated carbon is very wide, it is expensive. [10]. Activated carbon adsorbents are also nonselective. They are not recommended for lowering the levels of aqueous concentration below the required limits [11]. Due to their unique properties, mesoporous silica has attracted attention for application as adsorbent material or as part of adsorbent in nanocomposites polymer matrix. Mesoporous silica provides functionalizable surface, high surface area and consists of tailorable pore dimensions which make them good adsorbents [12]. Amine group impact positively on the performance of the adsorption systems of dyes, recalcitrant organic compounds and heavy metals enabling amination of MCM–41 (MCM–41–NH2) [13]. Mesoporous silica functionalized with amino group has been used for nitrate adsorption. Post-synthesis grafting method was used to synthesize mesoporous silica functionalized with amino group. Under reflux conditions, post-synthesis grafting method involves reacting organosilane using an appropriate solvent with surface silanol groups from mesoporous material synthesized previously [14].Adsorption efficiency of adsorbents is affected by factors such as pH, temperature, time and initial concentration of the pollutant [15]. The aim of this study was to determine the efficiency of diazonium silica in lowering the levels of nitrites in wetland water.
2. Materials and Methods
2.1. Materials
- Silica sand was used as raw material to prepare diazonium silica. Phosphorous pentachloride (PCl5), ethylenediamine (EDA), hydrochloric acid (HCl, 37%), dimethyl formamide (DMF), sodium hydroxide (NaOH), sodium nitrite, and methanol analytical grade were purchased from Sigma Aldrich and used without any further purification. All solutions were prepared using double deionized water.
2.2. Preparation of Diazonium Silica Adsorbent
- Synthesis of diazonium silica was carried out in three steps. The first step involved chlorination of raw silica. 2.0g of raw silica was put into 250cm3 three necked flask and 25 mL of DMF was added while stirring with magnetic stirrer. 1.5g of phosphorous pentachloride (PCl5) was added dropwise to the raw silica suspended in DMF. The mixture was refluxed for 2hrs, filtered and washed with deionized water then dried under vacuum at 70°C for 24hrs. The second step involved the amination of chlorinated silica. 5.0g of chlorinated silica was reacted directly with 25ml ethylenediamine. The resulting mixture was filtered, washed and dried in a desiccator for 48hrs [16]. In the last step 1.0g sample of aminated silica was dissolved in 20ml of water placed in 100ml conical flask. The solution was shaken vigorously and then placed in a beaker containing 25g of crushed ice. The resulting solution was reacted with 1.4g sodium nitrite placed in 3ml of water. The mixer was continuously shaken for a period of 5 minutes. The solution was allowed to stand with frequent shaking for 5 minutes. A solution of 5.2g of crystallized sodium acetate in 10ml was added where, a yellow precipitate of diazonuim silica began to form immediately. The precipitate was allowed to stand for 20 minutes with frequent shaking ensuring that the temperature does not exceed 20°C. Yellow diazonium silica was filtered on a Buchner funnel. The residue was washed with 100ml deionized water and dried at room temperature.
2.3. Characterization of Diazonium Silica Using FT-IR Spectroscopy
- FT-IR analysis was done for raw silica (RS), chlorinated silica (CS), aminated silica (AS) and diazonium silica (DS) [17].1.0 mg of each sample of RS, CS, AS and DS were mixed thoroughly with 50.0mg of potassium bromide (KBr). The mixture was pulverized and then pressed in a vacuum into pellets. The pellets were then put into spectrophotometer machine (FT-IR 8400 model Shimadzu Tokyo Japan) for analysis. The adsorbent spectra were measured in a wavenumber range of 4000cm-1 to 400cm-1 [18].
2.4. Adsorptive Process for Nitrites
- Adsorption capacity for nitrites using diazonium silica was studied at various parameters such as initial concentration, temperature, pH and contact time. Nitrite concentration was varied from 20ppm-60ppm. The absorbance was measured at 207 nm on Uv-Vis spectrometer. The effect of pH on the nitrite solution was investigated in the range of 2-7 adjusted using NaOH and HCl solutions with the aid of pH meter. Contact time was varied from 30-150 minutes and temperature varied from 25°C to 45°C. The adsorption experiments were carried out at a stirring speed of 150rpm.
2.5. Adsorption Isotherms, Kinetics and Thermodynamics
- Adsorption capacities for nitrites were investigated by adding 0.02g of diazonium silica to 100ml of nitrite solution separately and changing the initial concentration of the model solution at the optimum pH of 3.0. The agitation of the mixture was done at 150rpm for contact time 1hr at constant temperature of 30°C. The mixture was filtered and the amount of nitrites in the filtrate was analyzed using Uv-Vis. The entire process was done in triplicates, Amount of nitrites adsorbed by diazonium silica during the batch experiments were calculated by the expression in equation 1
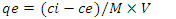 | (1) |
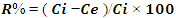 | (2) |
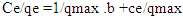 | (3) |
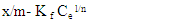 | (4) |
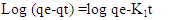 | (5) |
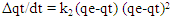 | (6) |
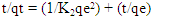 | (7) |
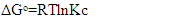 | (8) |
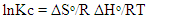 | (9) |
3. Results and Discussion
3.1. Characterization of Raw Silica, Chlorinated and Aminated Silica Material
- The FT-IR spectra bands found at 1051 and 794 cm-1 (Figure 1) are the characteristic anti-symmetric and symmetric stretching modes (Si-O-Si) of SiO4 [25]. The presence a small band found at 970cm-1 is allocated to Si-OH stretching mode and the band at 433cm-1 is assigned to Si-O-Si bending vibrations. The bands at 3435cm-1 and 1647cm-1 were assigned to the stretching and bending vibrations of the silanol groups (Si-OH) respectively.
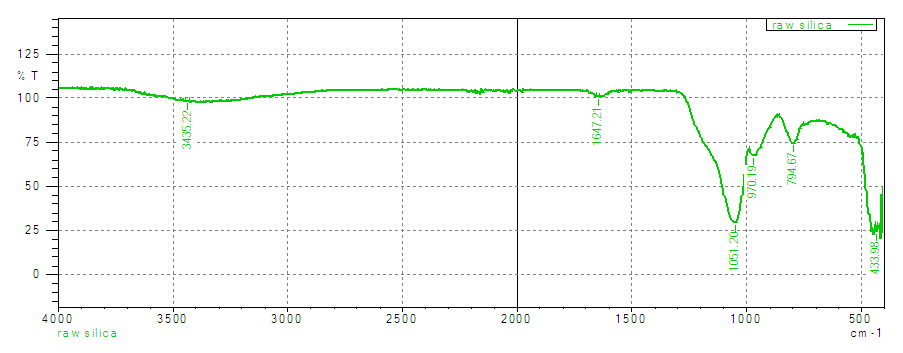 | Figure 1. Showing the FT-IR spectra of raw silica |
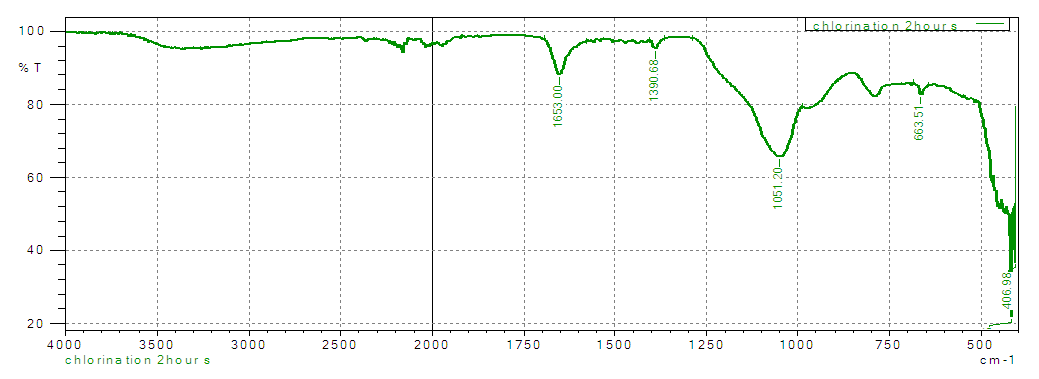 | Figure 2. FT-IR spectrum of chlorinated silica |
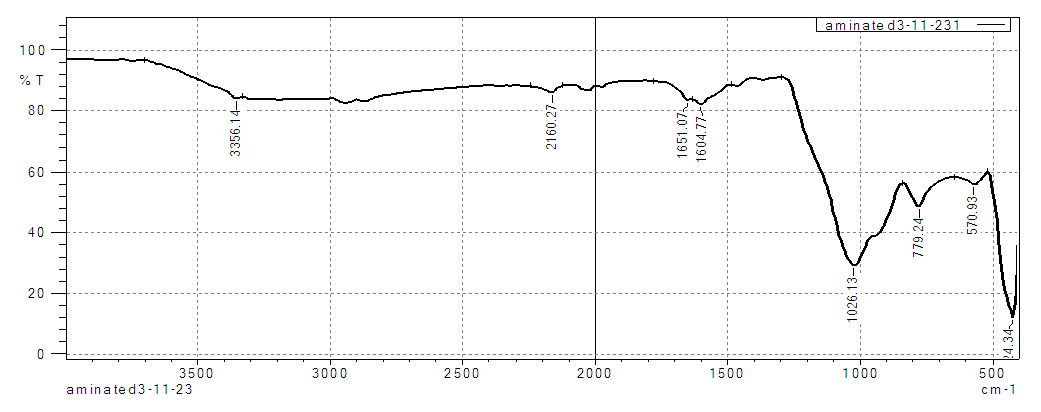 | Figure 3. FT-IR spectrum of aminated silica |
3.1.1. Overlaid FT-IR Spectra of Raw, Chlorinated and Aminated Silica
- The overlaid spectra of RS, CS and AS gives clear differences in the position of their functional groups. More pronounced peaks are observed in the chlorinated and the aminated silica. The observation’s confirms that ethylenediamine (secondary amine) was successfully anchored on the silica material. Similar results were reported when the following studies were carried during chlorination and amination of quartenized maize tassel [26].
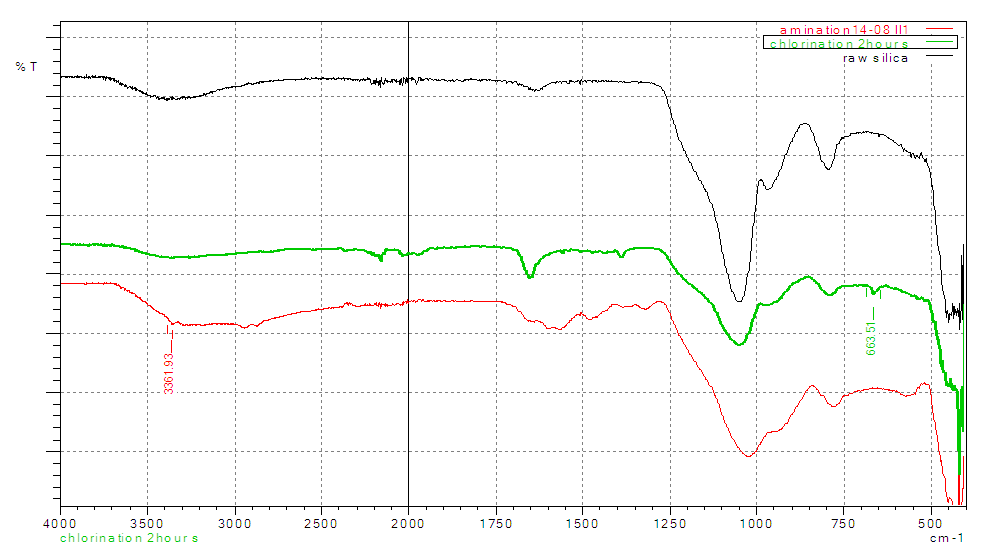 | Figure 4. FT-IR overlaid spectra of raw silica, chlorinated, and aminated silica |
3.1.2. FT-IR Characterization of Diazonium Silica
- The presence of a new open chain azo (N=N) group band in the region 1575cm-1 -1630cm-1 (Figure 5) confirms the successful synthesis of silica diazonium salt [28]. The bands found at 1049 and 791 cm-1 are the characteristic anti-symmetric and symmetric stretching modes (Si-O-Si) of SiO4 [27]. There is a shift of spectra band assigned to (Si-O-Si) shifting from 1051cm-1 in raw silica to 1049cm-1 in diazonium silica. The shift may be attributed to new electronic interactions during the formation of diazo group. The presence of nitrogen atoms can lead to changes in the electronic distribution in the silica structure affecting the energy levels of the electronic transitions. Shift can also be caused by the bond changes. The interaction between the silica framework and the diazo group can lead to the changes in band length and angles which can shift vibrational frequencies.
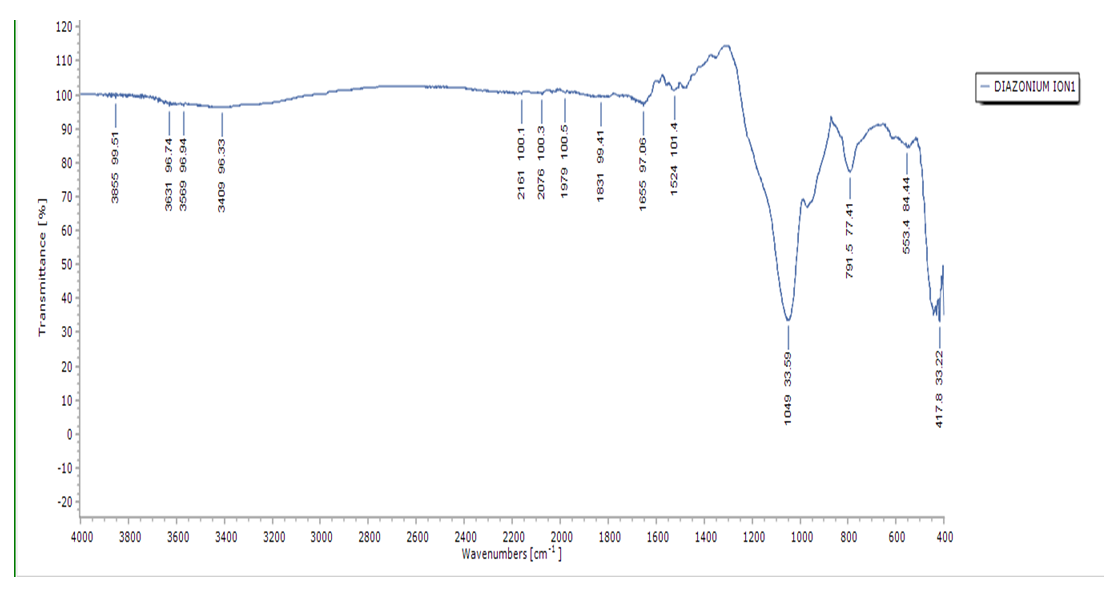 | Figure 5. FT-IR spectrum of diazonuim silica |
3.2. Effect of Temperature on the Adsorption of Nitrites
- The effect of temperature on the adsorption of nitrites ions into diazonuim silica was investigated (Figure 6). The amount of nitrite ions adsorbed into diazonium silica decreased with an increase in temperature from (298-318K) which suggest that the adsorption process was exothermic. The calculations of thermodynamic parameters further supported the results obtained.
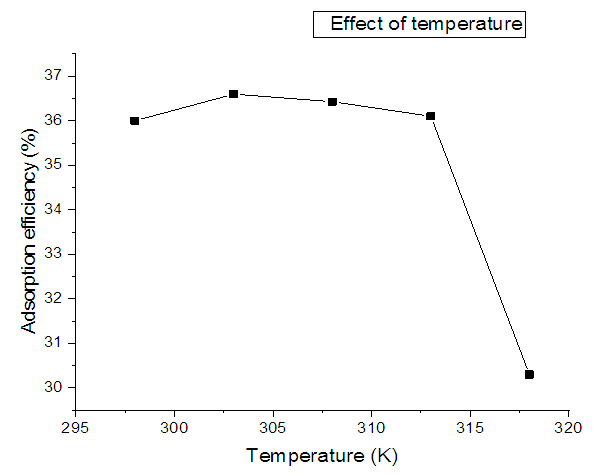 | Figure 6. Variation of adsorption efficiency against temperature |
3.3. Effect of pH on the Adsorption Efficiency of Nitrites
- The variation of pH was done from pH 2 to 7. It was observed that the optimal adsorption was achieved at pH 3 where the percentage removal of nitrites ions was 28.4. Effect of pH on the adsorption of nitrites ions into diazonuim silica is shown in Figure 7. From the results obtained it shows that there is no significant change in nitrites ions removal with the increase in pH. This indicates that diazonium silica can remove nitrite ions from aqueous solution over a wide range of pH [29]. A pH of 3 was selected as the optimal pH for more adsorption experiments in order to achieve suitable removal efficiency and nitrite removal capacity.
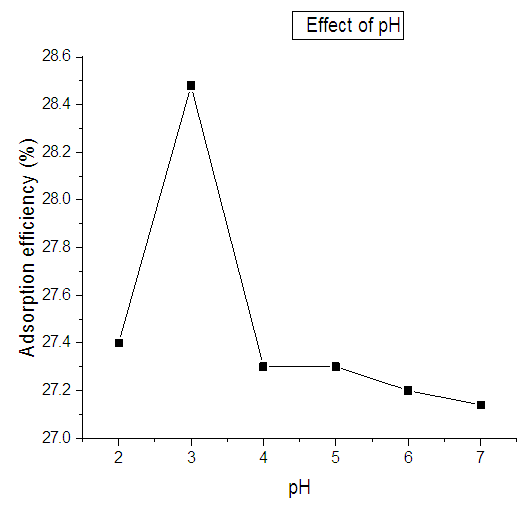 | Figure 7. Variation of adsorption of nitrites against pH using diazonium silica |
3.4. Effect of Initial Concentration
- The effect of initial concentration with adsorption efficiency is shown in Figure 8. Initial concentration was varied from 20ppm to 60ppm. Contact time, pH, temperature and adsorbent dose were kept constant. The results obtained showed the variation of efficiency with change in initial concentration ranging from 50.6% to 18% as shown graphically. It is observed that removal efficiency of nitrites ions first increased with increase in initial concentration and then decreased as the initial concentration increases. The decrease in the adsorption efficiency is caused by the increase in the initial concentration attributed by the saturation of adsorption sites. As the initial concentration of the adsorbate increases more adsorption sites on the adsorbents are occupied. When most of these sites are occupied there are fewer sites available for additional adsorbate molecules leading to a decrease in the percentage removal of the adsorbate from the solution. A decrease can also be caused by surface overcrowding.
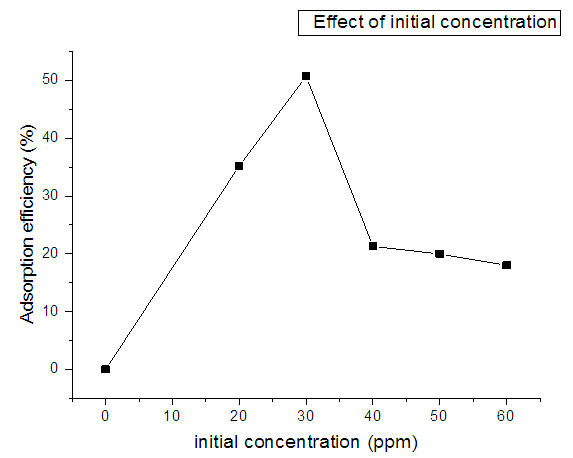 | Figure 8. Variation of adsorption of nitrites against initial concentration using diazonium silica |
3.5. Effect of Contact Time on the Adsorption of Nitrites
- Different contact time (30, 60, 90, 120, 150 and 180 minutes) were selected to conduct batch adsorption studies by taking initial concentration of nitrites as 30ppm. Other conditions were a dosage of 0.02g of the adsorbent at pH 3 and a temperature of 303K. The effect of contact time on the adsorption of nitrites onto diazonium silica is shown in Figure 9. The data reveals that as the contact time increases rate of adsorption first increases and the decreases to a point of becoming almost constant. This is caused by aggregation of nitrites ions with increase in contact time making it almost impossible to diffuse more into the adsorbent structure at higher energy sites. This makes the pores get filled up and start offering resistant to diffusion of aggregated nitrite ions into the adsorbent. The maximum adsorption is achieved at a period of 1 hour which is taken as the equilibrium time [30].
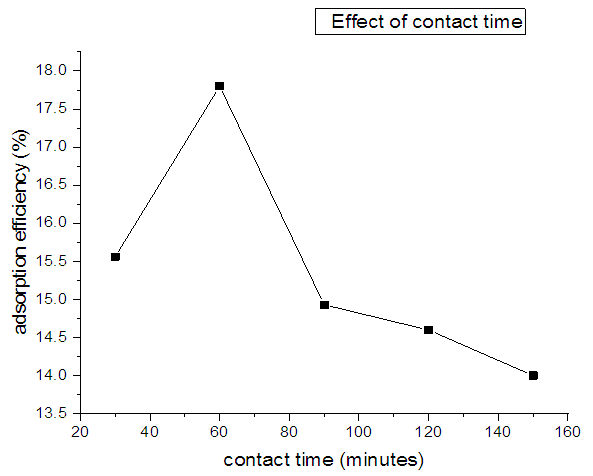 | Figure 9. Variation of adsorption of nitrites against contact time using diazonium silica |
3.6. Comparison of Efficacy of Diazonium Silica for Adsorption of Nitrite With Raw Silica as an Adsorbent
- From the analysis obtained it can be concluded that diazonium silica is a better adsorbent as compared to silica sand (Figure 10). This is indicated by high adsorption efficiency of diazonium silica. Silica sand is not typically used for adsorption of anions as it primarily consist silicon dioxide (SiO2) which has a neutral surface charge and does not have high affinity for anions.
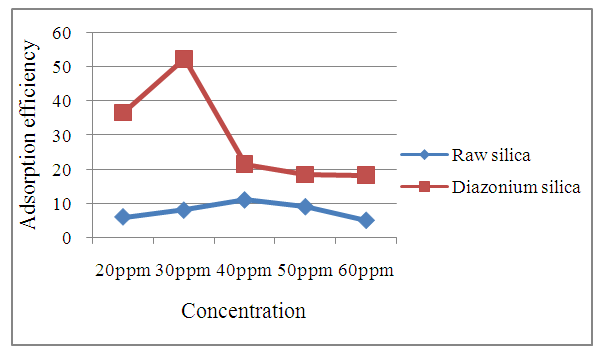 | Figure 10. Comparison of adsorption efficiency of raw silica with diazonium silica |
3.7. Adsorption Isotherm
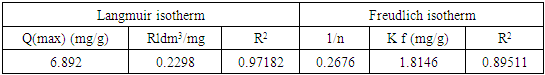
- The sorption equilibrium is frequently described using the Langmuir equation. The Langmuir isotherm model assumes that the uptake of metal anions takes place on the homogeneous surface using monolayer adsorption where there is no interaction between the adsorbed ions. The model also assumes that all the surface sites are alike and can accommodate one adsorbed molecule. Another assumption is that the adsorption process is reversible and the adsorbed molecule cannot migrate across the surface or interact with the neighboring molecule. In order to get the equilibrium data, the initial concentration of nitrites ions were varied keeping the adsorbent mass constant in each sample. Using the linearized Langmuir isotherm adsorption capacities and Langmuir constant were calculated. Adsorption capacities q(max) and Langmuir equilibrium constant kl were estimated from the slope and the intercept respectively from a plot of ce/qe versus ce (Figure 11). From the equation 3 adsorption capacity of nitrites (mg/g) obtained was 6.547 and kl was 0.07940. The separation factor Rl was found to between 0-1 indicating favaourable biosorption process. The correlation coefficient of Langmuir isotherm model R2 was 0.97029 as illustrated in Table 1 showing that there was a linear relationship or correlation [31].
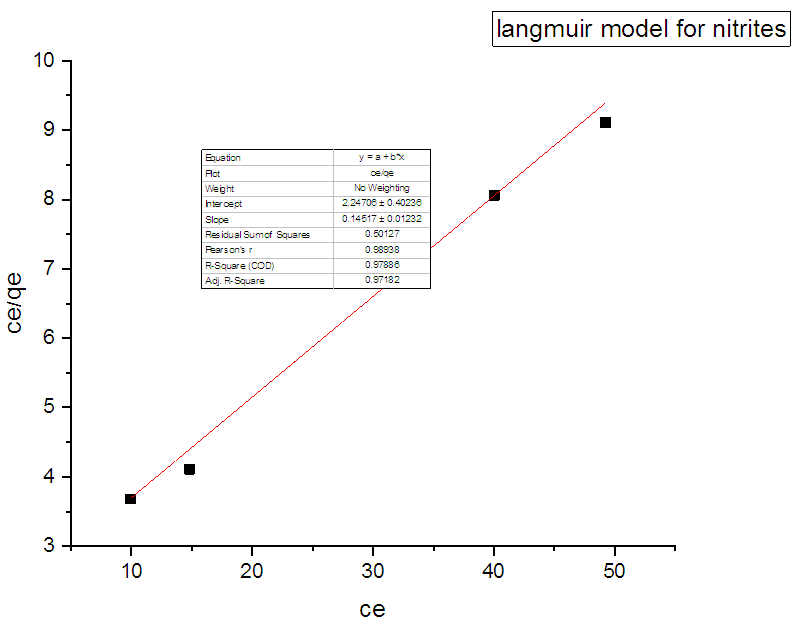 | Figure 11. Langmuir adsorption isotherm for adsorption of nitrites ions into diazonium silica |
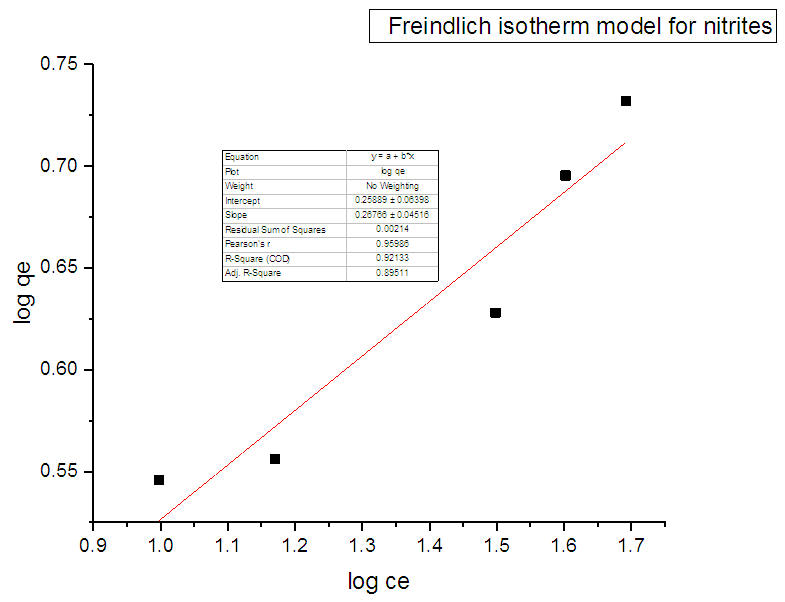 | Figure 12. Freindlich adsorption isotherm for adsorption of nitrites ions into diazonium silica |
|
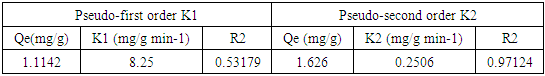
3.8. Adsorption Kinetics of Diazonuim Silica
- From the results recorded in Table 2 for the adsorption of nitrites in the diazonium silica adsorbent showed an excellent compliance with the pseudo-second order better than pseudo-first order. According to their respective correlation coefficient R2 value, R2 value obtained from pseudo-first order is 0.53179 which is lower than R2 obtained from pseudo -second order which is 0.97124. The calculated equilibrium capacities were closer to the experimental ones in the pseudo-second order. The findings obtained from the study indicates that the adsorption kinetics of nitrites on diazonium silica perfectly followed the pseudo-second order model [24].
|
3.9. Thermodynamic Parameters
- The process of adsorption of nitrites into diazonium silica was studied using varying temperatures (298, 303, 308, 313 and 318K). Thermodynamic parameters ΔG0 were calculated according to equation 8 and ΔH0 and ΔS0 were calculated from Vant hoff linear plot according to the equation 9. Results obtained were as illustrated in figure 13 for adsorption of nitrites ions into diazonuim silica. The value of ΔH0 was -21.591KJmol-1. The negative value indicated that the interaction of nitrites adsorbed into diazonium silica was exothermic in nature [24]. The value of ΔS0 was found to be 55.113Jmol-1. The positive value suggests a spontaneous process. This is due to redistribution of energy between the adsorbate and the adsorbent. An increase in randomness at the solid-solution interface during the adsorption process enhances increase in entropy leading to overall positive ΔS0 [33].
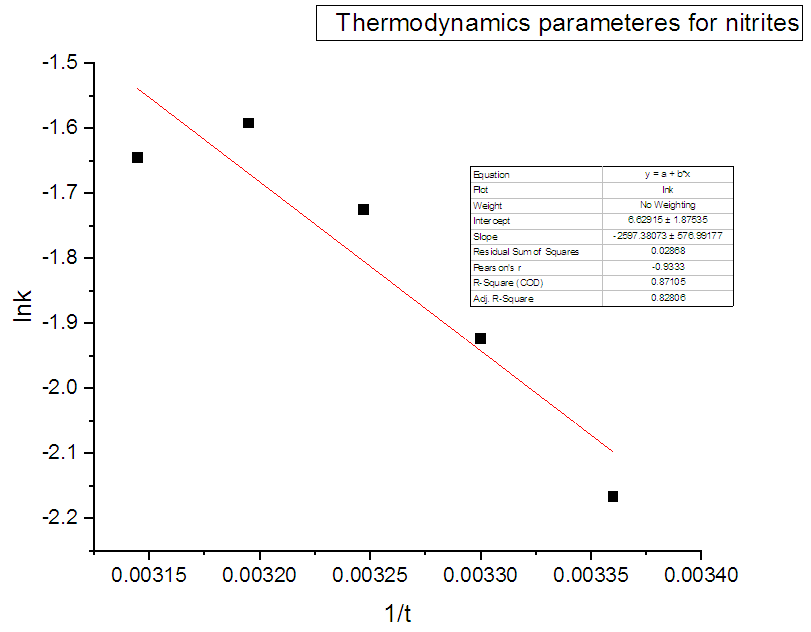 | Figure 13. Adsorption thermodynamic of nitrites ions into diazonuim silica |
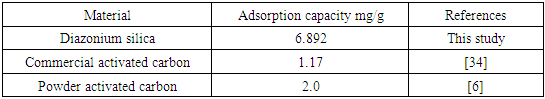
3.10. Comparison of Adsorption Efficiency of Commercial Activated Carbon with Diazonium Silica Adsorbent
- Table 3 compares the adsorption capacity of diazonium silica adsorbent with different materials used for adsorption nitrites. The results indicate that adsorption efficiency of diazonium silica prepared in this study is higher than earlier reported values.
|
4. Conclusions
- During the study diazonium silica was prepared and used to lower the levels of nitrites in wetland waters. Characterization analysis reveals that diazonuim silica contains unique properties as an efficient adsorbent as compared to raw silica material. Functional groups and thermal stability were improved when silica was functionalized with amine. The optimal experimental conditions for achieving maximum nitrites removal were as follows: pH 3.0, initial nitrite concentration 20ppm, contact time 60 minutes, optimal temperature of the solution 303K and shaking speed 150rpm. Equilibrium time course for anions uptake and thermodynamics analysis were performed. Equilibrium data was described by Langmuir and Freindlich adsorption isotherms. The maximum sorption capacity for nitrites were recorded as 16.45mg/g. Langmuir isotherm model was well obeyed according to the correlation coefficient R2= 0.97182. Pseudo second order kinetic model was best obeyed in the adsorption of nitrites in diazonium silica. Thermodynamic parameters revealed that the sorption of nitrites in diazonium silica was exothermic in nature. The present measurements shows the feasibility of diazonium silica with different characteristics as potential sorbent for nitrites.
Conflict of Interest
- The authors declare that they have no conflict of interest.
ACKNOWLEDGEMENTS
- Special thanks to the Mr. Antony Njagi laboratory technician of Chemistry Department, Kenyatta University, for helping in providing relevant glassware, chemicals and reagents used during the study. Thanks to all staff members of Chemistry Department, Kenyatta University for assist me in various ways.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML