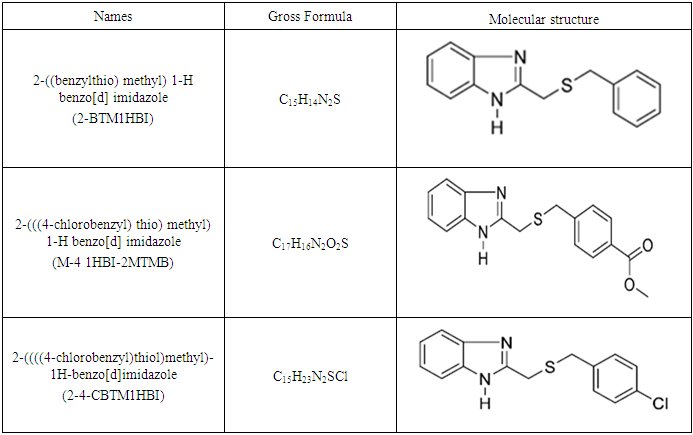
| [1] | Arrousse, N., Fernine, Y., Haldhar, R., Berdimurodov, E., Hamza Ichou, Al-Zaqri, N., Mohammed, K., Seong-Cheol, K., Mustapha, T., 2023, Corrosion protection studies of different alloys in 1 M HCl by benzimidazole derivative: Combined molecular dynamic simulations/DFT, Journal of Environmental Chemical Engineering, 11(3), 109642. |
| [2] | Caid, Z.A.E., Left, D.B., Thoume, A. et al., 2023, A Comprehensive Computational Study of N-Phenylacetamide Derivatives as Corrosion Inhibitors for Copper: Insights from DFT and Molecular Dynamics, Journal of Bio- and Tribo-Corrosion, 9, 83. |
| [3] | Matine, A., Es-Sounni, B., Bakhouch, M. et al., 2024, Design, synthesis, and evaluation of a pyrazole-based corrosion inhibitor: a computational and experimental study, Scientific Reports, 14, 25238. |
| [4] | Da,Y., Liu, Y., Baimei, T., Tengda, M., Shihao, Z., Yazhen, W., Lei, G., Baohong, G., Yangang, H., 2021, Theoretical and electrochemical analysis on inhibition effects of benzotriazole derivatives (un- and methyl) on copper surface, Journal of Molecular Structure, 1243, 130871. |
| [5] | Jinbo, J., Baimei, T., Shihao, Z., Tengda, M., Lei, G., Wei L., Mei, Y., Fangyuan, W., Haoyu, D., Xiaolong, W., 2022, Investigation on the control effect of benzotriazole and two derivatives on cobalt pitting corrosion in chemical mechanical polishing process: A combination of experiments and theoretical simulations, Journal of Molecular Liquids, 367, Part B, 120487. |
| [6] | Abdelmalek, M., Mohamed, B., Haydar Mohammad-Salim, H., Abdallah, M. E., Ali H. et al., 2024, Exploring the synthesis and application of a pyrazole derivative in corrosion protection: Theoretical modeling and experimental investigations, Journal of Molecular Structure, 1312, Part 1, 138458. |
| [7] | Madkour, L.H., Elshamy, I.H., 2016, Experimental and computational studies on the inhibition performances of benzimidazole and its derivatives for the corrosion of copper in nitric acid, International Journal of Industrial Chemistry 7, 195-221. |
| [8] | Fouda, A.S., Ismail, M.A., Khaled, M.A. et al., 2022, Experimental and computational chemical studies on the corrosion inhibition of new pyrimidinone derivatives for copper in nitric acid. Scientific Reports, 12, 16089. |
| [9] | Deyab, M.A., AlGhamdi, J.M., Abdeen, M.M. et al. (2024), Chemical, electrochemical, and quantum investigation into the use of an organophosphorus derivative to inhibit copper corrosion in acidic environments. Scientific Reports, 14, 11395. |
| [10] | Sourav, K. S., Abhiram, H., Naresh Chandra, M., Priyabrata, B., 2016, A comparative density functional theory and molecular dynamics simulation studies of the corrosion inhibitory action of two novel N-heterocyclic organic compounds along with a few others over steel surface, Journal of Molecular Liquids, 215, 486-495. |
| [11] | Hsissou, R., About, S., Berisha A., Mohamed, B., Mohammed, A., Najat, H., Ahmed, E., 2019, Experimental, DFT and molecular dynamics simulation on the inhibition performance of the DGDCBA epoxy polymer against the corrosion of the E24 carbon steel in 1.0 M HCl solution, Journal of Molecular Structure, 1182, 340-351. |
| [12] | Shahraki, M., Maryam, D., Shirin, E., 2016, Theoretical studies on the corrosion inhibition performance of three amine derivatives on carbon steel: Molecular dynamics simulation and density functional theory approaches, Journal of the Taiwan Institute of Chemical Engineers, 62, 313-321. |
| [13] | Laihemdi, F., Barhoumi, A., Zarri, M. et al., 2024, Inhibition of corrosion of an aluminum alloy by rosemary and eucalyptus extracted oils in 1 M hydrochloric acid medium: an experimental and theoretical study, Environmental Science and Pollution Research, 31, 62147-62173. |
| [14] | Anusuya, N., Saranya, J., Benhiba, F. et al., 2022, Isoxazoline Derivatives as Inhibitors for Mild Steel Corrosion in 1M H2SO4: Computational and Experimental Investigations. Journal of Materials Engineering and Performance, 31, 7204–7219. |
| [15] | Dahmani, K., Galai, M., Ouakki, M. et al. 2023, New Xanthene Diones Compounds as a Corrosion Inhibitor of Mild Steel in Acid Medium: Electrochemical, Surface Characterization and Theoretical Insights. Chemistry Africa, 6, 2049–2069. |
| [16] | Sourav, K.S., Alokdut D., Pritam, G., Dipankar, S., Priyabrata, B., 2016, Novel Schiff-base molecules as efficient corrosion inhibitors for mild steel surface in 1 M HCl medium: experimental and theoretical approach; Physical Chemistry Chemical Physics, 18, 17898-17911. |
| [17] | Frisch, M. J., Trucks, G. W., Schlegel, H. B. et al., 2003, Gaussian 03, Gaussian, Inc.: Pittsburgh P A. |
| [18] | Becke, A.D. (1993) Density-Functional Thermochemistry. III. The Role of Exact Exchange. Journal of Chemical Physics, 98, 1372-1377. |
| [19] | Lee, C., Yang, W. and Parr, R.G. (1988), Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density, Physical Review B, 37, 785-789. |
| [20] | Lei, G., Savaş Kaya, Ime Bassey, O., Xingwen, Z., Yujie, Q., 2017, Toward understanding the anticorrosive mechanism of some thiourea derivatives for carbon steel corrosion: A combined DFT and molecular dynamics investigation, Journal of Colloid and Interface Science, 506, 478-485. |
| [21] | Shubhra, P., Deepti, J., Shamima, H., Amrita, B., Shrivastava, R., Saroj, K. P., Hemanta, K. K., Hassane L., Ill-Min C., Debasis B., 2019, A new insight into corrosion inhibition mechanism of copper in aerated 3.5 wt.% NaCl solution by eco-friendly Imidazopyridine Dye: experimental and theoretical approach, Chemical Engineering Journal, 358, 725-742. |
| [22] | Beda, R. H. B., Niamien, P. M., Avo Bilé, E. B., Trokourey, A., 2017, Inhibition of Aluminum Corrosion in 1.0 M HCl by Caffeine: Experimental and DFT Studies, Hindawi Advances in Chemistry, 6975248. |
| [23] | Tigori, M. A.; Kouyaté, A.; Kouakou, V.; Niamien, P. M.; Trokourey, A. 2020, Computational Approach for Predicting the Adsorption Properties and Inhibition of Some Antiretroviral Drugs on Copper Corrosion in HNO3, European Journal of Chemistry., 11, 235-244. |
| [24] | Garcia-Ochoa, E., Guzmán-Jiménez, S.J. Hernández, J.G., Pandiyan, T., Vásquez Pérez, J.M., Cruz-Borbolla, J., 2016, Journal of Molecular Structure, 1119, 314–324. |
| [25] | Pearson RG., 1988, Absolute Electronegativity and Hardness: application to Inorganic Chemistry, Inorganic Chemistry, 27(4), 734-740. |
| [26] | Tigori M.A, Koné A., Koua, N. R., Yeo M., Niamien, P.M., 2022, Copper corrosion inhibition in nitric acid solution by 2-(1,3-dihydrobenzimidazol-2-ylidene) -3-oxo-3-(pyridin-3-yl) propanenitrile: Gravimetric, Quantum chemical and QSPR studies. Mediterranean Journal of Chemistry, 12(2), 123-139. |
| [27] | Quadri, T.W., Olasunkanmi, L. O., Fayemi, O. E., Lgaz, H., Dagdag, O., Sherif, E. M., Alrashdi, A. A., Akpan, E. D., Lee, H., Ebenso, E. E., 2022, Computational insights into quinoxaline-based corrosion inhibitors of steel in HCl: Quantum chemical analysis and QSPR-ANN studies, Arabian Journal of Chemistry, 15(7), 103870. |
| [28] | Lukovits, I., Shaban, A., & Kalman, E. (2003). Corrosion inhibitors: Quantitative structure–activity relationships, Russian Journal of Electrochemistry, 39, 177-181. |
| [29] | Khattabi, M., Benhiba, F., Tabti, S., Djedouani, A., El Assyry, A., Touzani, R., Warad, I., Oudda, H., Zarrouk, A., 2019, Performance and computational studies of two soluble pyran derivatives as corrosion inhibitors for mild steel in HCl, Journal of Molecular Structure, 1196, 231-244. |
| [30] | Hsissou, R, About, S., Zaki, S., Fouad, B., Nuha Wazzan, N., Lei G., Nouneh, K., Samir, B., Hamid, E., Mohamed E.T., Mohammed, A., Ahmed, E., 2021, Synthesis and anticorrosive properties of epoxy polymer for CS in [1 M] HCl solution: Electrochemical, AFM, DFT and MD simulations, Construction and Building Materials, 270, 121454. |
| [31] | Dagdag, O., El Harfi, A., Cherkaoui, O., Safi, Z., Wazzan, N., Guo, L., Akpan, E., Verma, C., Ebenso, E., Jalgham, R.T., 2019, Rheological, electrochemical, surface, DFT andmolecular dynamics simulation studies on the anticorrosive properties of newepoxy monomer compound for steel in 1 M HCl solution, RSC Advances, 9, 4454–4462. |
| [32] | Dagdag, O., El Harfi, A., El Gouri, M., Safi, Z., Jalgham, R.T., Wazzan, N., Verma, C., Ebenso, E., Kumar, U.P., 2019, Anticorrosive properties of Hexa (3-methoxy propan-1, 2-diol) cyclotri-phosphazene compound for carbon steel in 3% NaCl medium: gravimetric, electrochemical, DFT and Monte Carlo simulation studies, Heliyon, 5, e01340. |
| [33] | Turuvekere, K. C., Kikkeri, N. M., Doddahosuru, M. G., Harmesh, C. T., 2016, Inhibition activity of new thiazole hydrazones towards mild steel corrosion in acid media by thermodynamic, electrochemical and quantum chemical methods, Journal of the Taiwan Institute of Chemical Engineers, 67, 521-531. |
| [34] | Khattabi, F. Benhiba, S. Tabti, A. Djedouani, A. El Assyry, R. Touzani, I. Warad, H. Oudda, A. Zarrouk, 2019, Performance and computational studies of two soluble pyran derivatives as corrosion inhibitors for mild steel in HCl, Journal of Molecular Structure, 1196, 231-244. |
| [35] | El Faydy, M., Benhiba, F., Lakhrissi, B., Ebn Touhami, M., Warad, I., Bentiss, F., Zarrouk, A., 2019, The inhibitive impact of both kinds of 5-isothiocyanatomethyl-8-hydroxyquinoline derivatives on the corrosion of carbon steel in acidic electrolyte, Journal of Molecular Liquids, 295, 111629. |
| [36] | Lei, G., Savaş, K., Obot, I.B., Xingwen, Z., Yujie, Q., 2017, Toward understanding the anticorrosive mechanism of some thiourea derivatives for carbon steel corrosion: A combined DFT and molecular dynamics investigation, Journal of Colloid and Interface Science, 506, 478-485. |
| [37] | Gazquez, J. L., Cedillo, A., Vela, A., 2007, Electrodonating and electroaccepting powers, The Journal of Physical Chemistry, 111, 1966-1970. |
| [38] | Hegazy, M.A., Atlam, F.M., 2016, Three novel bolaamphiphiles as corrosion inhibitors for carbon steel in hydrochloric acid: Experimental and computational studies, Journal of Molecular Liquids, 218, 649-662. |
| [39] | Mounir, G., Chtita S., Hmamouchi, R., Adad, A, Mohammed B., Lakhlifi T., 2016, The inhibitory activity of aldose reductase of flavonoid compounds: Combining DFT and QSAR calculations, Journal of Taibah University for Science, 10(4), 534-542. |
| [40] | Masego, D., Lukman O. O., Omolola, E.F., Sasikumar, Y., Ramaganthan, B., Bahadur, I., Abolanle, S. A., Mwadham, M. K., Eno, E.E., 2015, Some Phthalocyanine and Naphthalocyanine Derivatives as Corrosion Inhibitors for Aluminium in Acidic Medium: Experimental, Quantum Chemical Calculations, QSAR Studies and Synergistic Effect of Iodide Ions, Molecules, 20, 15701-15734. |



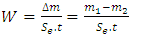
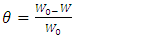
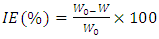
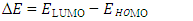


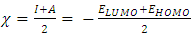
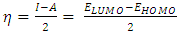
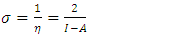
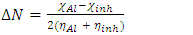
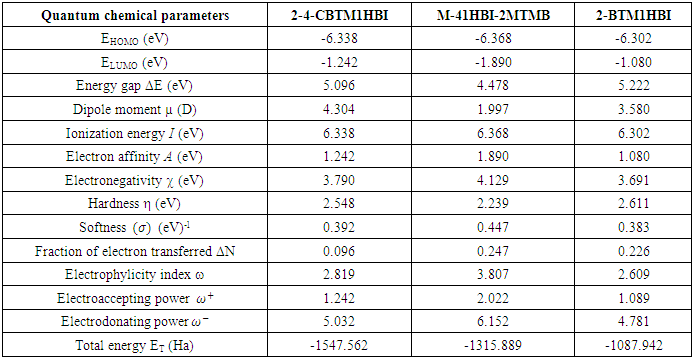
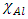 and
and 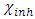 denote the absolute electronegativity of copper and inhibitor molecule respectively. The calculations were performed using the following theoretical values:
denote the absolute electronegativity of copper and inhibitor molecule respectively. The calculations were performed using the following theoretical values: 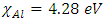 [25] and
[25] and 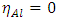 [25]
[25]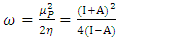
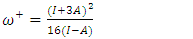
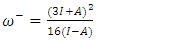
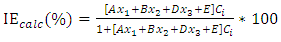
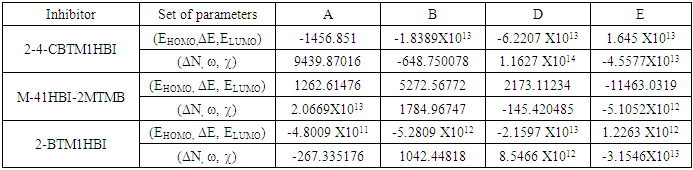
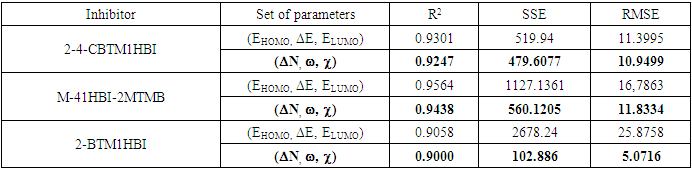
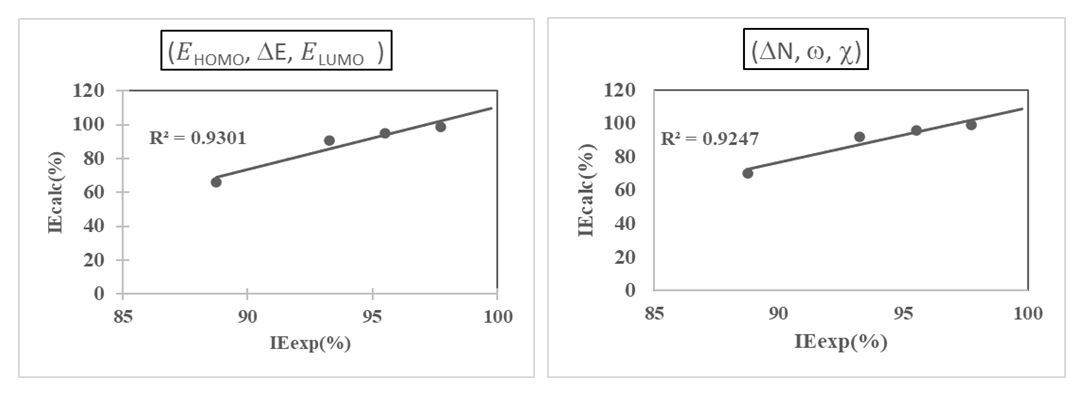
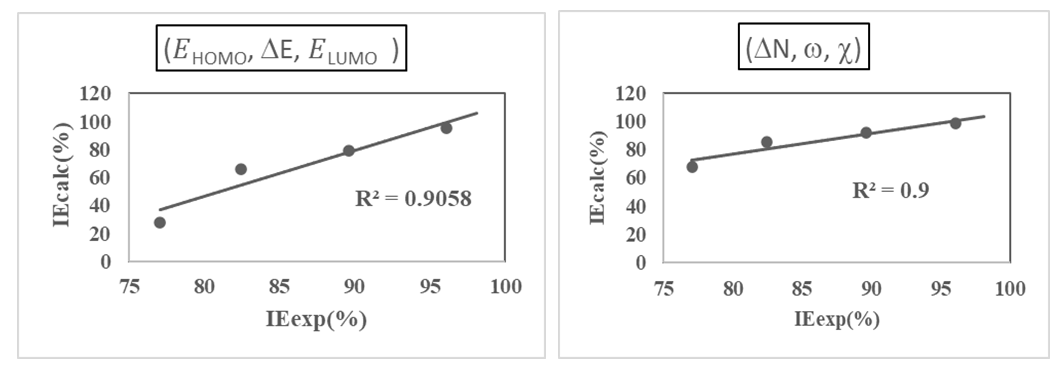
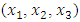 were tested to analyze the data obtained. This approach results in a system of four equations with four unknowns: A, B, D and E. The aim is to determine the values of the coefficients A, B, D and E for each molecule, so that the theoretical inhibition efficiency corresponds as closely as possible to that measured experimentally. The calculations required to solve this system were carried out using EXCEL software.
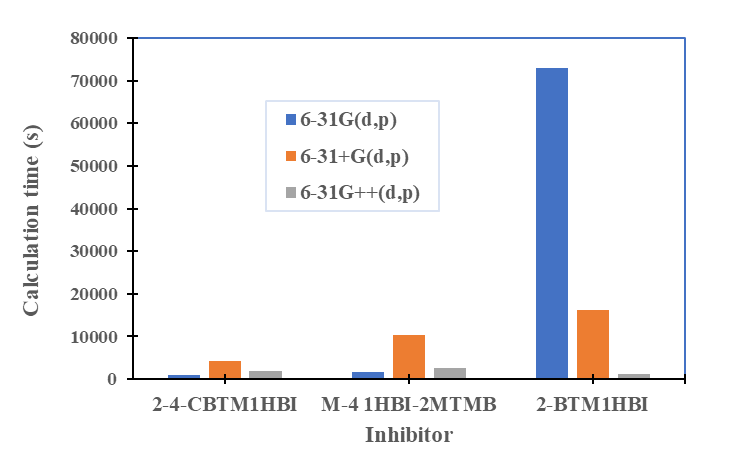
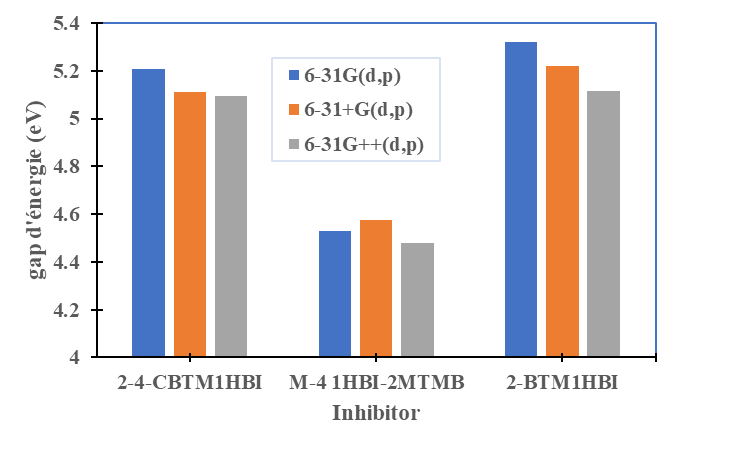
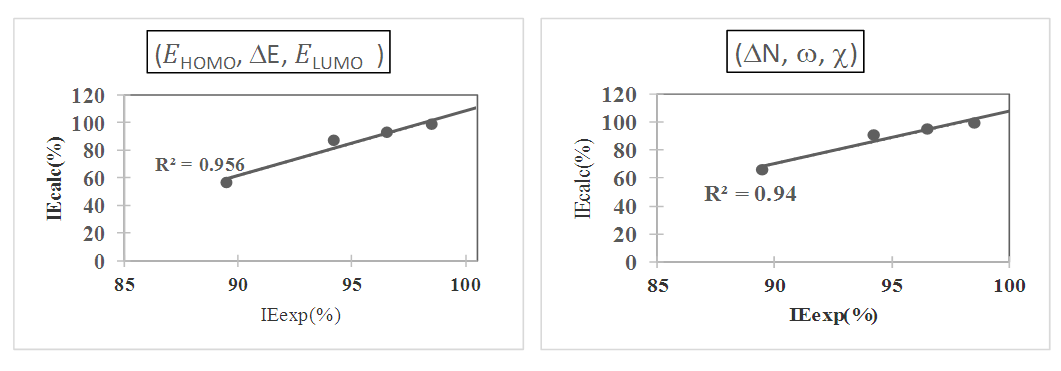
were tested to analyze the data obtained. This approach results in a system of four equations with four unknowns: A, B, D and E. The aim is to determine the values of the coefficients A, B, D and E for each molecule, so that the theoretical inhibition efficiency corresponds as closely as possible to that measured experimentally. The calculations required to solve this system were carried out using EXCEL software. 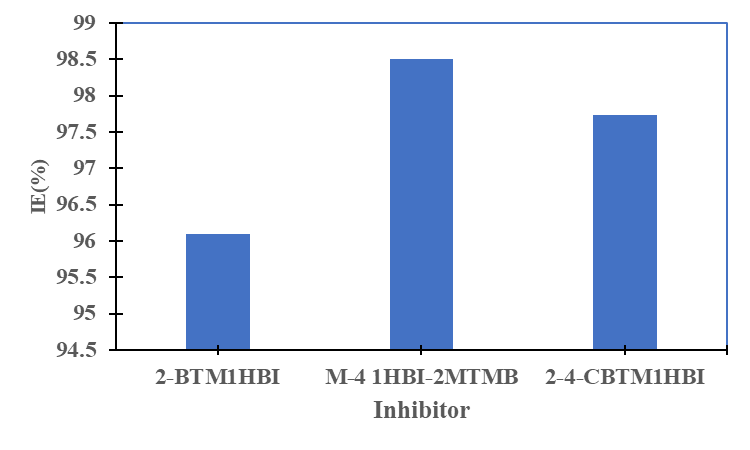
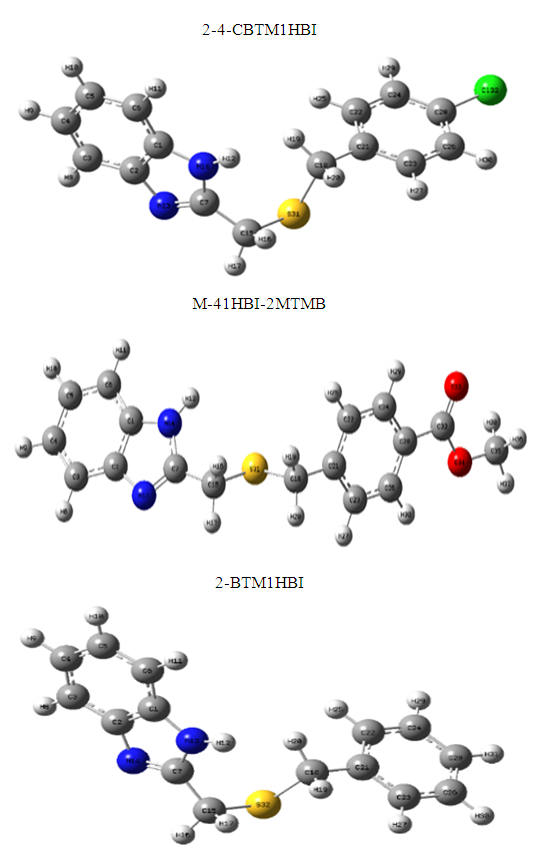
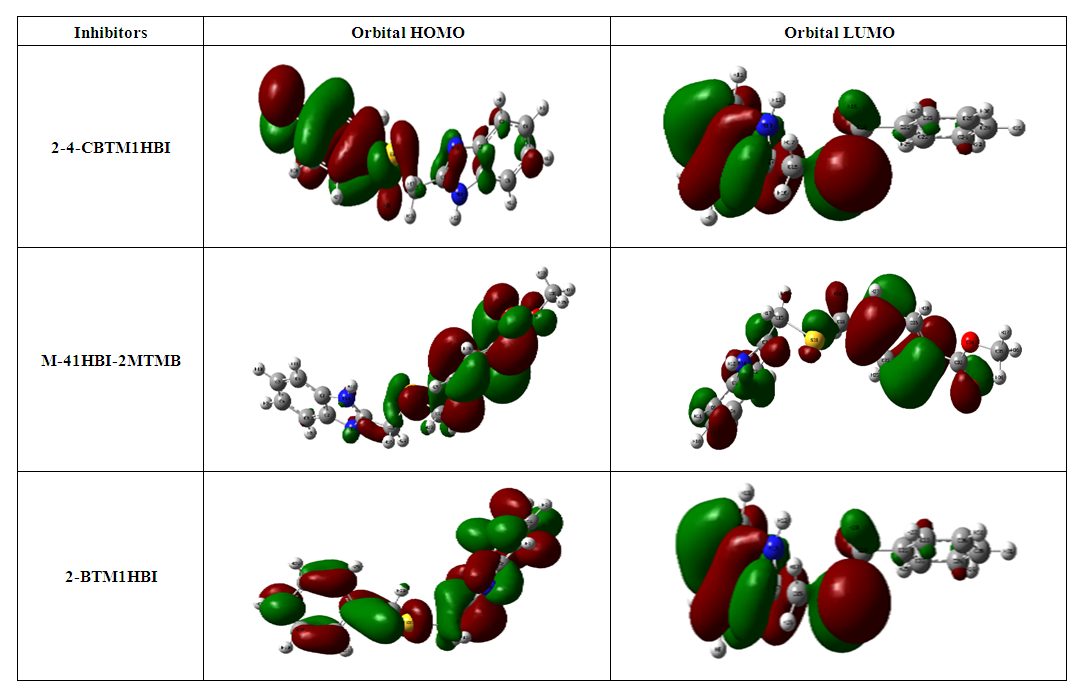


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML