-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2024; 14(5): 55-62
doi:10.5923/j.chemistry.20241405.02
Received: Nov. 29, 2024; Accepted: Dec. 26, 2024; Published: Dec. 28, 2024

Antiradical and Antidiabetic Potential of Newbouldia laevis (P. Beauv.) ex Bureau Leaf Extracts: Inhibition of α-Glucosidase Activity
Rawendé Sédégo1, Bakouan Youssoufou1, Téeda Hamidou Ganamé2, Jules Yoda3, Benjamin Ouédraogo2, 3, Bintou Sessouma1, Karifa Bayo1
1Laboratory of Molecular and Materials Chemistry (LC2M), University Joseph KI-ZERBO, Ouagadougou, Burkina Faso
2Laboratory of Environmental and Bio-Organic Analytical Chemistry (LCAEBiO) University Joseph KI-ZERBO, Ouagadougou, Burkina Faso
3Department of Traditional Medicine, Pharmacopoeia – Pharmacy (MEPHATRA-PH), Research Institute for Health Sciences (IRSS /CNRST), Ouagadougou, Burkina Faso
Correspondence to: Bintou Sessouma, Laboratory of Molecular and Materials Chemistry (LC2M), University Joseph KI-ZERBO, Ouagadougou, Burkina Faso.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

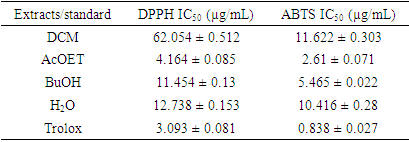
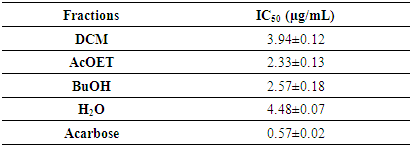
This study aimed to evaluate the therapeutic potential of extracts from the leaves of Newbouldia laevis (P. Beauv.) ex Bureau, a plant used in the management of diabetes. In this study, the different analyses focused on the anti-radical and anti-diabetic activities of selected extracts. Antidiabetic activity was studied by monitoring the inhibitory effect of the fractions on α-glucosidase, using the in vitro model. Anti-radical activities were evaluated by DPPH and ABTS methods. Ethyl acetate and butanol fractions showed better inhibitory effects against 𝛂-glucosidase enzymatic activity, with IC50 values estimated at 2.33 ± 0.13 µg/mL and 2.57 ± 0.18 µg/mL, respectively, compared to acarbose, whose IC50 is estimated at 0.57 ± 0.02 mg/mL. For free radical scavenging activity, the same fractions showed the highest DPPH● radical scavenging with IC50 values of 4.164 ± 0.085 and 11.454 ± 0.13 μg/mL, respectively. For the cationic ABTS●+ radical, the IC50 values are 2.61 ± 0.071 and 5.465 ± 0.022 μg/mL, respectively. These results suggest that Newbouldialaevis leaf extracts have significant anti-diabetic properties.
Keywords: Newbouldia laevis, α-glucosidase, Antiradical, Anti-diabetic activity
Cite this paper: Rawendé Sédégo, Bakouan Youssoufou, Téeda Hamidou Ganamé, Jules Yoda, Benjamin Ouédraogo, Bintou Sessouma, Karifa Bayo, Antiradical and Antidiabetic Potential of Newbouldia laevis (P. Beauv.) ex Bureau Leaf Extracts: Inhibition of α-Glucosidase Activity, American Journal of Chemistry, Vol. 14 No. 5, 2024, pp. 55-62. doi: 10.5923/j.chemistry.20241405.02.
Article Outline
1. Introduction
- Diabetes is a chronic condition in which blood sugar levels are very high because the body cannot longer produce enough insulin or use it effectively [1]. There are three (03) main types of diabetes, including gestational diabetes, insulin-dependent diabetes (type 1 diabetes) and non-insulin-dependent diabetes (type 2 diabetes or diabetes mellitus). The latter is the most common, accounting for an estimated 90% of diabetes cases [2]. Many studies have shown that oxidative stress is a contributing factor in the development and progression of many diseases, including diabetes [3]. These studies have investigated the effect of oxidative stress on the onset and development of diabetic disorders via the formation of free radicals, which are likely to prevent the normal functioning of insulin and thus induce the onset or progression of diabetes mellitus [4-6]. The precariousness of diabetes care, the severity of the disease and its socio-economic impact make it a major public health problem in African countries, particularly in Burkina Faso [7]. Studies show that approximately 80% of the population in these countries rely on traditional herbal medicine for primary health care [8-10]. Medicinal plants are therefore of great interest as a potential source for the development of phytomedicines for many diseases in low-income populations. Many recipes derived from medicinal plants are proposed by phytotherapists for the treatment of diabetes, for their pharmacological properties in the regulation of oxidative stress and in the management of diabetes. Such is the case with Newbouldia laevis (P. Beauv.) ex Bureau, known as African hyssop, which is traditionally used in the treatment of diabetes. Studies have shown that it contains compounds of biological interest such as phenolic derivatives, flavonoids and steroids with anti-free radical properties [11-12]. Other studies have highlighted the contribution of these compounds to anti-diabetic activity, and their presence inhibits α-glucosidase, which is responsible for the hydrolysis of polysaccharides [13]. The aim of this study is to evaluate the in vitro antiradical and anti-diabetic activities of Newbouldia laevis leaf extracts.
2. Materials and Methods
2.1. Plant Material
- The plant material consists of leaves of Newbouldia laevis (P. Beauv.) ex Bureau, harvested in August 2022 in the city of Ouagadougou. The plant was identified at the Laboratory of Plant Biology and Ecology of the University Joseph KI-ZERBO. A specimen was deposited in the university herbarium with identification number 18017. The harvested leaves were thoroughly washed and dried in an airy room, away from sunlight and dust (for 2 weeks), then ground to powder using a blade grinder.
2.2. Extraction
- The plant was extracted according to the protocol described by Karanga et al. with some modifications [14]. In fact, 100 g of the plant powder was degreased with hexane to remove chlorophyll and wax. After filtration, the pomace was dried, and 50 g were macerated for 72 hours in 500 mL of a hydroalcoholic solvent (ethanol-water 80/20 v/v). After filtration, the filtrate obtained was concentrated with a rotary evaporator to a minimum volume and then lyophilized to obtain a dry crude extract. 9 g of this crude extract was redissolved in 150 mL of distilled water, followed by liquid-liquid extraction by successive exhaustions with solvents of increasing polarity, such as dichloromethane (DCM), ethyl acetate (AcOET) and n-butanol (BuOH). All partitions were repeated in triplicate and the different fractions obtained were concentrated to dryness and stored appropriately for further analysis.
2.3. Antioxidant Potential of Selective Extracts
2.3.1. DPPH Method
- The DPPH° test measures the free radical scavenging capacity of pure molecules or plant extracts in a model system (organic solvent, room temperature) according to the method of Rodríguez et al. with some modifications [15]. It measures the ability of an antioxidant (RH, usually phenolic compounds) to reduce the chemical radical DPPH° by hydrogen transfer. DPPH°, which is initially violet in color, is converted to DPPH-H, which is pale yellow in color (Figure 1). The DPPH method is performed as follows 1 mL of extract (appropriately diluted) is mixed with 4 mL of freshly prepared methanolic DPPH solution. After incubation for 10 min, the absorbance is measured at 517 nm using a microplate spectrophotometer. The radical scavenging activity (IC50) is expressed in µg/mL.
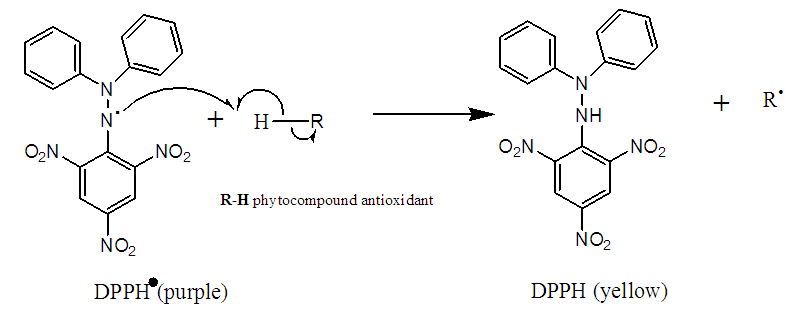 | Figure 1. Reduction of DPPH by an antioxidant |
2.3.2. FRAP Method
- The FRAP assay was performed according to the method of Piljac-Zegarac J. et al. with some modifications [16]. To prepare the FRAP reagent, 0.312 g of detripyridyltriazine (TPTZ) is dissolved in 100 mL of 40 mM HCl solution. This results in a solution with a final TPTZ concentration of 10 mM. At the same time, prepare a sodium acetate buffer solution. Dissolve 2.56 g sodium acetate in 5.36 mL acetic acid (80% pure) and make up to 250 mL with distilled water. Prepare a 20 mM ferric chloride (FeCl3; 6H2O) solution by dissolving 0.54 g FeCl3 in 100 mL of distilled water. Finally, the TPTZ-acetate buffer-FeCl3 solutions are mixed at a ratio of 1/10/1 (v/v/v) to obtain the FRAP reagent ready for use in the assays. Read the absorbance at 595 nm.
2.3.3. ABTS Radical Reduction Method
- This test is based on the redox mechanism of ABTS (ammonium salt of 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid). In this test, the ABTS salt loses an electron to form a dark-colored cation radical (ABTS.+) in solution. In the presence of the antioxidant, this radical is reduced to the ABTS+ cation, resulting in a discoloration of the solution (Figure 2). The method used is that described by Arts (2004), RE et al. (1999) [17-18]. 19.2 mg of ABTS was dissolved in 5 mL of distilled water. 3.312 mg potassium persulfate was added and the mixture was kept in the dark at room temperature for 12 to 16 hours. 4.5 mL of this mixture was then diluted in 220 mL of analytical methanol. A series of twelve (12) successive dilutions is made starting from the parent solution concentration of the samples (1 mg/mL).
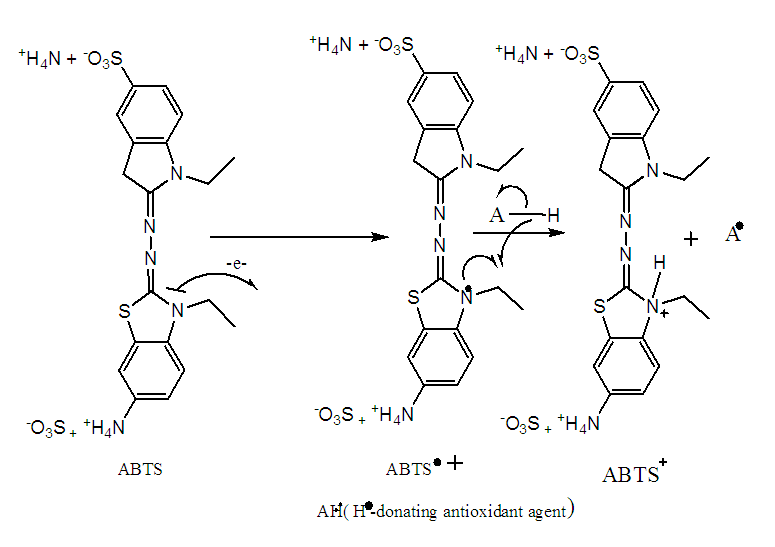 | Figure 2. ABTS oxidation-reduction mechanism |
2.3.4. Determination of Compounds with Antioxidant Potential
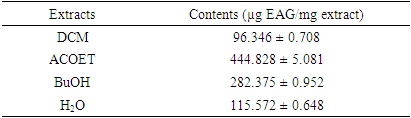
- The content of phenolic compounds in a crude extract is measured by the Folin-Ciocalteu method [19-20]. The principle is to oxidize all phenolic compounds with the Folin-Ciocalteu reagent, using gallic acid as a calibration standard. The total phenolic compound (TCP) content of different fractions of Newbouldia laevis leaves was measured using a colorimetric method with slight modifications of the Folin-Ciocalteu method. 1 mL of extract or gallic acid was mixed with 1 mL of diluted Folin-Ciocalteu reagent. After eight minutes at room temperature in a cube, 2 mL of a saturated solution of sodium carbonate (7.5% in water) was added to the mixture. After thirty minutes in the dark at 37°C, the absorbance of the blue solution obtained was measured using a UV-visible spectrophotometer at 760 nm. The results are obtained by plotting the absorbance readings against a previously established calibration curve using gallic acid as a reference and are expressed as µg gallic acid equivalent (GAE) per milligram of crude extract (µg GAE/mg extract).
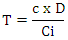 T = Content in µg Gallic Acid Equivalent per mg extract C = Sample concentration read from standard curve D = Dilution factor of the sample to be assayed Ci = initial concentration of the sample solution to be assayed.
T = Content in µg Gallic Acid Equivalent per mg extract C = Sample concentration read from standard curve D = Dilution factor of the sample to be assayed Ci = initial concentration of the sample solution to be assayed.2.4. Determination of α-Glucosidase Inhibitory Activity
- The inhibition of the fractions on α-glucosidase activity was assessed using the chromogenic method reported by Ranilla et al. (2010) [21] with slight modifications. For this purpose, a mixture containing 20 µL of α-glucosidase (1 unit/mL), 120 µL of 0.1 M phosphate buffer pH 6.9 and 10 µL of each plant fraction at different concentrations is introduced. Each solution obtained is pre-incubated in 96-well microplates at 37°C for 15 minutes. After incubation, the enzymatic reaction is initiated by adding 20 µL of 5 mM p-nitrophenyl-α-D-glucopyranoside in 0.1 M phosphate buffer (pH 6.9) and mixing. The reaction mixture is incubated at 37°C for a further 15 minutes. Stop the reaction by adding 80 µL of 0.2 M sodium carbonate solution. Read the absorbance at 405 nm on a spectrophotometer. The reaction system without plant extracts is used as a control and the system without α-glucoside is used as a blank to correct for background absorbance. Acarbose is used as a positive control. The rate of α-glucosidase inhibition is calculated using the following formula:
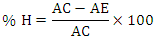 % H = Percentage of inhibitionAC = Control absorbance AE = Absorbance of sample
% H = Percentage of inhibitionAC = Control absorbance AE = Absorbance of sample3. Results and Discussion
3.1. Antioxidant Activity
3.1.1. DPPH and FRAP Methods
- The antioxidant activity was assessed using the different fractions. The analysis combined the DPPH and FRAP methods. The results of the estimation of antioxidant activity by these two methods are presented in histogram form and expressed in µg TE/mg extract (Figure 3).
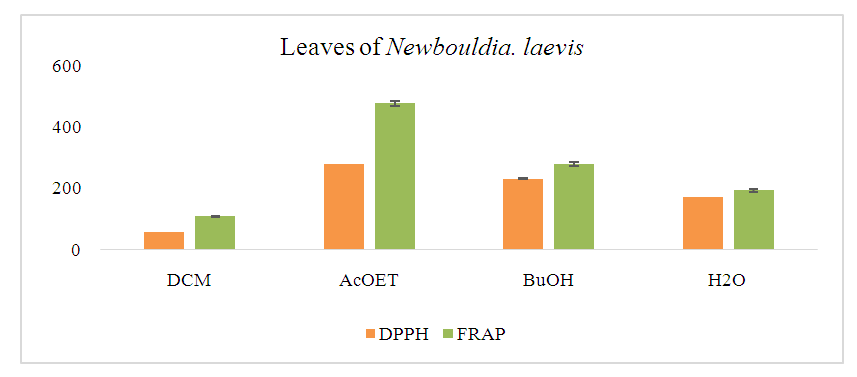 | Figure 3. Histogram of antioxidant content of fractions by DPPH and FRAP methods |
3.1.2. Anti-Free Radical Activity
- To substantiate the antioxidant properties of the different fractions, their free radical scavenging properties were assessed using the DPPH and ABTS methods. For this purpose, the 50% inhibition concentrations (IC50) of the different fractions were determined and the results are shown in the table below (Table 1).
|
3.1.3. Phenolic Compounds Content of Extracts
- The results obtained by evaluating the total phenolic compound (TPC) content of the leaf fractions of the studied plant are presented in Table 2, expressed as µg EAG/mg extract.
|
3.2. Inhibitory Activity of α-Glucosidase
- The anti-diabetic activity of the leaf extracts was tested using the different fractions. The figures below show the results obtained for α-glucosidase inhibition (Figures 4-6). They also show the evolution of the percentage of inhibition as a function of the concentration of the different selective extracts.
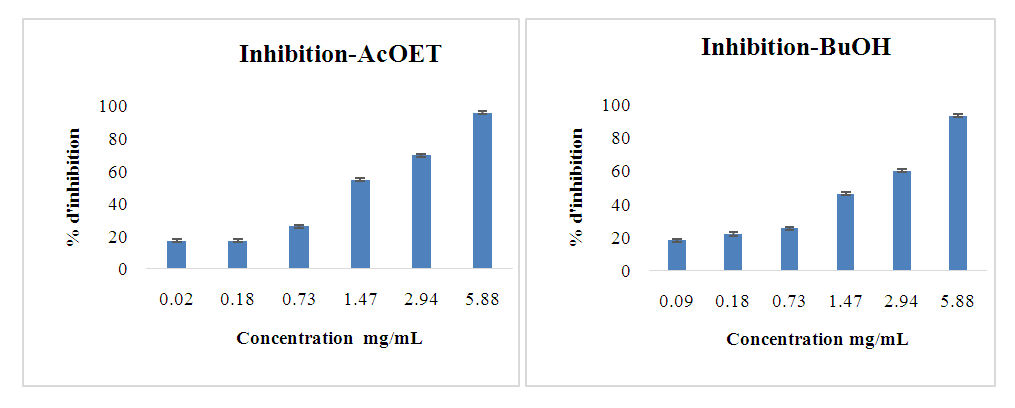 | Figure 4. Inhibitory activities of AcOET and BuOH extract fractions against α-glucosidase |
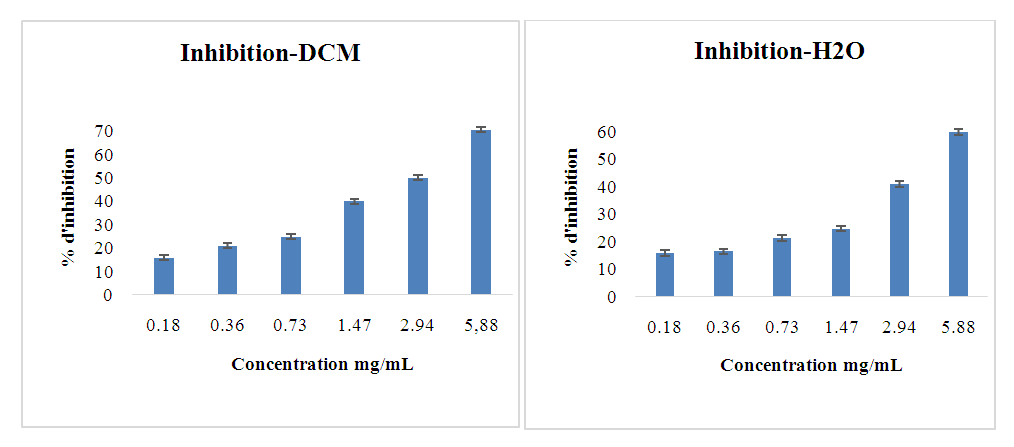 | Figure 5. Inhibitory activity of DCM and H2O extract fractions against α-glucosidase |
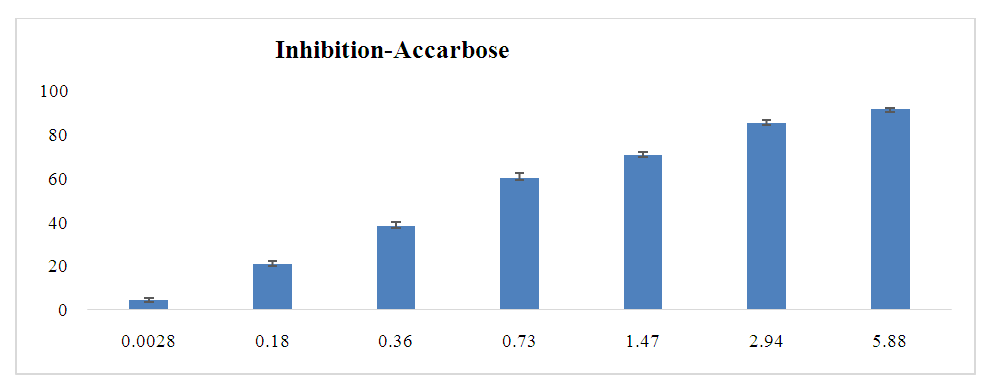 | Figure 6. Inhibitory activity of acarbose against α-glucosidase |
|
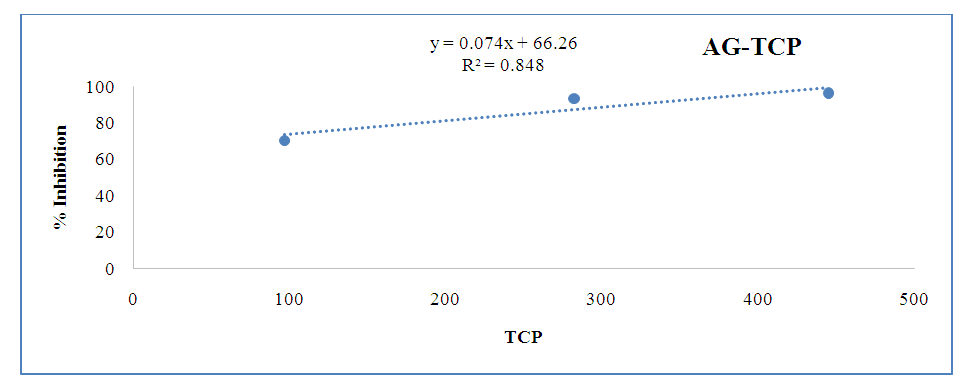 | Figure 7. Correlation between phenolic compounds and anti-diabetic activity |
4. Conclusions
- This study focused on the antioxidant potential and anti-diabetic activity of Newbouldia laevis leaf extracts. To determine the anti-diabetic activity, the inhibitory effects of the fractionated extracts on the enzyme α-glucosidase were examined. The results of the analyses showed that the AcOET and BuOH fractions were of interest, with better antioxidant and anti-diabetic activities. The antioxidant and anti-diabetic activity studies highlighted the inhibitory effect against α-glucosidase and the considerable anti-free radical activity of Newbouldia laevis leaves. These results provide a scientific support for the traditional use of this plant in the treatment of diabetes and could serve as a prerequisite for the development of phytomedicines.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML