-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2024; 14(4): 41-44
doi:10.5923/j.chemistry.20241404.01
Received: Jul. 1, 2024; Accepted: Aug. 2, 2024; Published: Aug. 17, 2024

Inquiry-Based Forensic Chemistry Lab: Detection of Common White Powders Using Bare Glassy Carbon Electrodes as Optimum Sensors
Suzanne Lunsford1, Papa Kofi Damte Andoh1, Kayla N. Clark2, Riley Rutkowski1
1Wright State University, Dept. of Chemistry, Dayton, Ohio, USA
2Wright State University, Dept. of Chemistry, Dayton, OH, USA
Correspondence to: Papa Kofi Damte Andoh, Wright State University, Dept. of Chemistry, Dayton, Ohio, USA.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

An inquiry-based lab experiment was developed to encourage undergraduate students to determine and identify the three unknown white powders utilizing bare carbon electrode versus gold or platinum working electrodes as a simple and efficient electrochemical sensor. Differential Pulse Voltammetry (DPV) was utilized to study and determine the electrochemical behavior of three white powders. The three white powders analyzed in solution were ascorbic acid (AA), paracetamol (PA), and caffeine (CA) without the need of prior separation utilizing a bare glassy carbon electrode. The lab was successful in introducing students to carbon electrodes as a cost -effective sensor material to utilize in the electrochemical DPV method to detect AA, PA and CA simultaneously without prior separation versus utilizing Au and Pt as working electrodes which also exhibited the potential to detect these white powders but has limitations such as cost and inability to perform simultaneous detection of more than two analytes.
Keywords: Electrochemical Sensor, Detection by Differential Pulse Voltammetry, Forensics, Unknown white powders
Cite this paper: Suzanne Lunsford, Papa Kofi Damte Andoh, Kayla N. Clark, Riley Rutkowski, Inquiry-Based Forensic Chemistry Lab: Detection of Common White Powders Using Bare Glassy Carbon Electrodes as Optimum Sensors, American Journal of Chemistry, Vol. 14 No. 4, 2024, pp. 41-44. doi: 10.5923/j.chemistry.20241404.01.
Article Outline
1. Introduction
- The forensic lab was developed to engage undergraduate students in the analysis of three white powders that are part of the food quality aspect, these three powders were caffeine (CA), ascorbic acid (AA) and paracetamol (PA). A bare glassy carbon electrode material was utilized to detect the three white solids by Differential Voltammetry (DPV) due to the glassy carbon having positive features of: chemical inertness, low cost and wide anodic potential window. Novel technological developments in the area of forensics, food quality and safety for products rely on equipment-based systems such as capillary electrophoresis, gas chromatography, high pressure liquid chromatography, infrared spectroscopy, Raman spectroscopy, nuclear magnetic resonance and UV-spectroscopy. [1-6]. These techniques require in-depth sample preparation before the analysis and are extremely expensive instruments that require highly trained workers. Therefore, electrochemistry methods have intrigued many researchers’ interest due to the simplicity, low cost, minimal pre-sample treatment, fast analysis time and high sensitivity. In this present lab, undergraduate students’ results will show the successful detection utilizing a glassy carbon electrode as the optimum bare electrode sensor to detect all three compounds AA, PA and CA by DPV without the need to prior separation [7]. This lab experiment has required two lab periods (total of 5-6 hours) for successful results due to learning the DPV instrumentation procedures and preparation of the working electrodes, and preparation of AA, PA, CA and mixture samples in buffer solution. Learning outcomes required students to utilize the DPV instrumentation in the optimum range to detect the three white powders and express the oxidation and reduction reactions for each of the samples: PA, AA and CA. Also, students were required to determine which working electrode (C, Au, Pt) could detect all three mixtures without the need of prior separation by DPV.
2. Experimental
2.1. Materials
- Glassy carbon electrode, bare gold electrode, bare platinum electrode, auxiliary platinum electrode, silver/silver chloride electrode, were all purchased from Bioanalytical Systems. Ascorbic acid, paracetamol, caffeine, and phosphate buffer were supplied by Merck company. Phosphate buffer pH at 7.4 was purchased from MilliporeSigma to dilute the white solids PA, CA, AA.
2.2. Equipment
- A three-electrode single compartment cell was utilized for the voltammetry studies with the platinum foil as the auxiliary, and Ag/AgCl/3 M NaCl (MF-2074, BAS) as the reference electrode. Bioanalytical Systems Epsilon potentiostat-galvanostat instrument was utilized to carry out the Differential Pulse Voltammetry using the following parameters: Step Potential, 4 mV and pulse width, 50 m/s, pulse period 200 m/s and pulse amplitude 10 mV. The scan rate is determined by Step E/pulse period, and this was determined to be 20 mV/s. Starting potential -200 mV and ending 1600 mV.
2.3. Electrode Preparation
- Electrodes were polished with 1.0μm alumina micro polish powder containing particles that offer very efficient removal rates to obtain smooth finish. Polished electrodes were soaked in 0.1 M phosphate buffer for about 30minutes before used for CPV experiments.
3. Results and Discussion
- Differential Pulse Voltammetry (DPV) was utilized as the electrochemical technique due to being a quantitative method that can assist in determination of specific species. Thus, DPV is a very powerful electroanalytical technique that allows for detection and quantification of a variety of species at low concentrations. The analytes were electroplated onto the working glassy carbon electrode during a deposition step and oxidized from the working electrode during the stripping step. The oxidation of AA, CA and PA leads to the release of protons as illustrated in the schemes below [8-10].
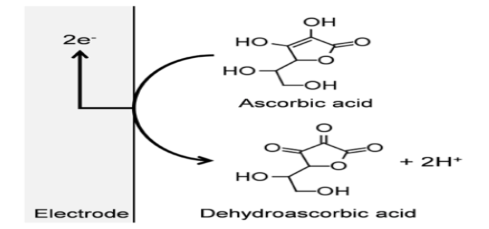 | Figure 1. Scheme of oxidation mechanism of AA |
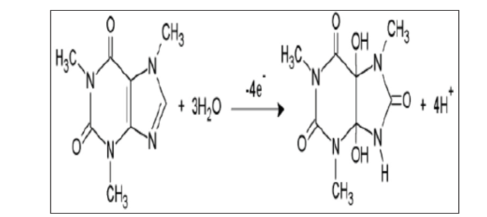 | Figure 2. Scheme of oxidation mechanism of CA |
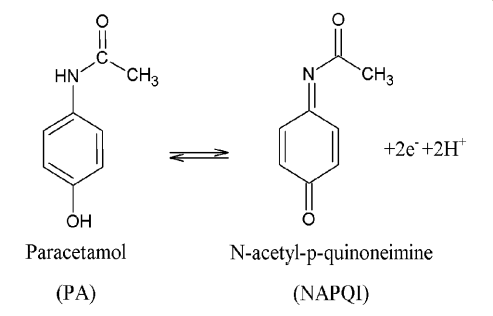 | Figure 3. Scheme of oxidation mechanism of PA |
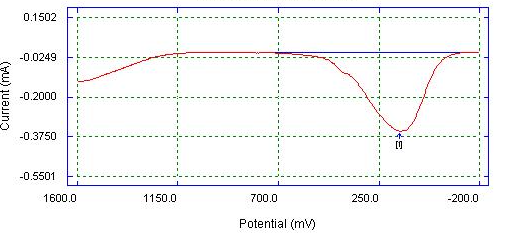 | Figure 4. DPV of Bare Glassy Carbon electrode detection of 0.05 M ascorbic acid in 0.1 M phosphate buffer |
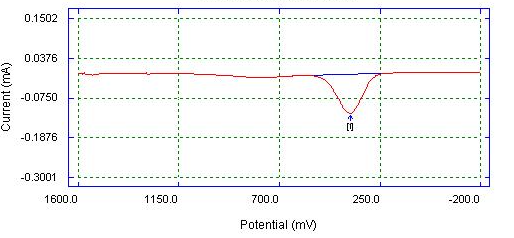 | Figure 5. DPV of Bare Carbon electrode detection of 0.05 M paracetamol in 0.1 M phosphate buffer |
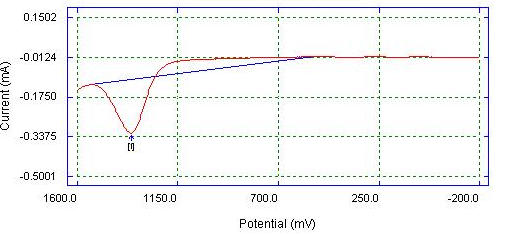 | Figure 6. DPV of Bare Glassy Carbon electrode detection of 0.05 M caffeine in 0.1 M phosphate buffer |
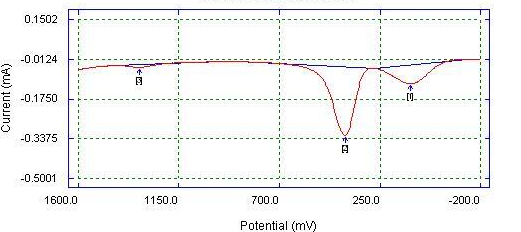 | Figure 7. DPV of Bare Glassy Carbon electrode detection of mixture (0.05 M AA, 0.05 M PA, 0.05 M CA) in 0.1 M phosphate buffer |
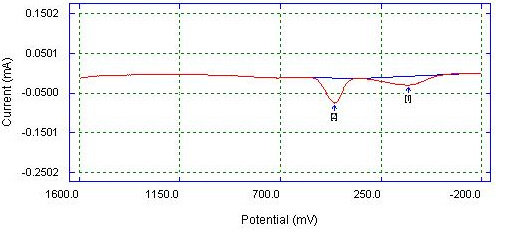 | Figure 8. DPV of Bare Platinum electrode detection of mixture (0.05 M AA, 0.05 M PA, 0.05 M CA) in 0.1 M phosphate buffer |
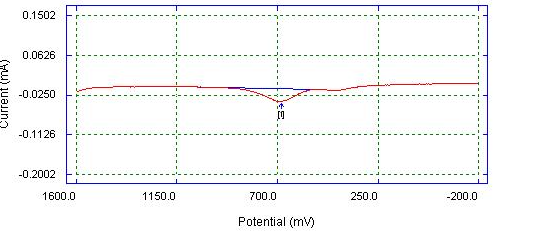 | Figure 9. DPV of Bare Gold electrode detection of mixture (0.05 M AA, 0.05 M PA, 0.05 M CA) in 0.1 M phosphate buffer |
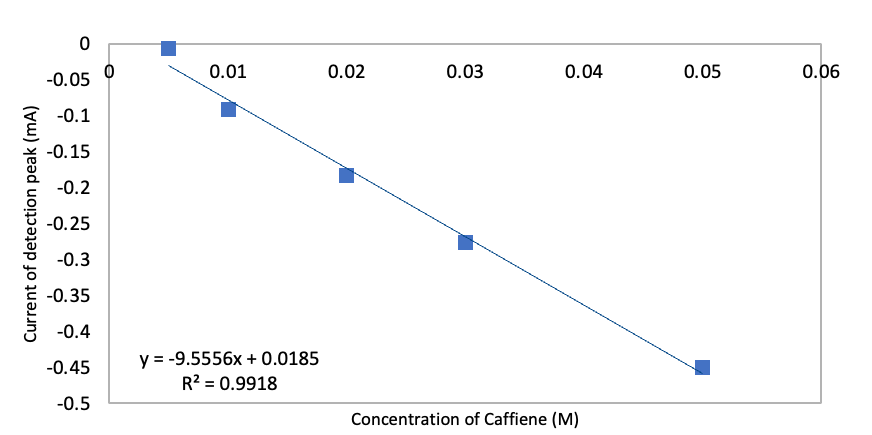 | Figure 10. Plot of Current versus Concentration for Glassy Carbon electrode to detect caffeine from 5X10-2 M to 5X10-4 M range in 0.1 M phosphate buffer |
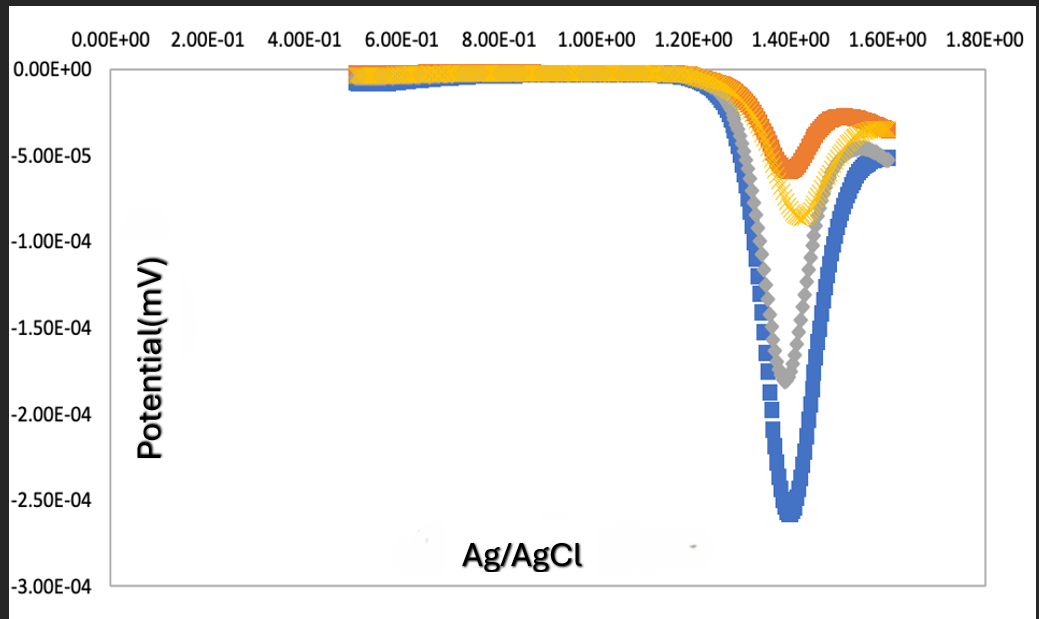 | Figure 11. Overlay -detection of caffeine by DPV, 5X10-2M - 5X10-4M in 0.1 M phosphate buffer |
4. Conclusions
- The glassy carbon electrode successfully detected the AA, PA and CA without the need of prior separation, no tedious sample preparation and fast analysis time. The stability of the glassy carbon electrode in the detection of caffeine can be shown by the detection at various concentrations thus no significant shift in the detection of caffeine, 1400 mV peak signal overall with a linear regression of 0.9918. DPV has been shown to be an effective way to detect unknown white powders based on the potential peak signal and the glassy carbon electrode has shown to be the most reliable working electrode to utilize when there is an overall mixture of AA, PA, and CA. Our students have found this lab to be informative in understanding how electrochemistry can be simple, provide low cost, minimal pre-sample treatment, fast analysis time and high sensitivity compared to other analytical techniques. However, as students learned in the literature that there may be the need to modify the bare electrodes with nanoparticles for additional unknown white solids to be analyzed and detected successfully by DPV to enhance the catalytic ability of the working electrode [11].
ACKNOWLEDGEMENTS
- Special thanks to Wright State University for their financial support provided by the Wright State University Chemistry Department and Students First Fund.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML