-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2023; 13(4): 102-106
doi:10.5923/j.chemistry.20231304.02
Received: Jul. 13, 2023; Accepted: Jul. 28, 2023; Published: Sep. 26, 2023

Investigation of the 3-Monochloropropane-1,2-Diol Levels in Edible Cooking Fats and Oils. A Case Study of the Kenyan Market
Phyllyis Kiende1, Alex King’ori Machocho1, Muriira Geoffrey Karau2
1Department of Chemistry, Kenyatta University, Nairobi, Kenya
2Kenya Bureau of Standards, Nairobi, Kenya
Correspondence to: Phyllyis Kiende, Department of Chemistry, Kenyatta University, Nairobi, Kenya.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Edible cooking oils which are solids or liquids at room temperature are either extracted or processed through chemical processes from various sources. During chemical preparation, contaminants such as 3-Monochloropropane-1,2-diol (3-MCPD) esters could result. 3-MCPD esters are formed during deodorization process as a result of high temperature and low moisture conditions in presence of chloride ion. This compound has been classified as carcinogenic and therefore of significant health concern. In Kenya, various refined oils are in the market for consumer use and their use is regulated by the standards or technical regulations which sets limits for various parameters. There is no data on the regulatory levels of 3-MCPD esters in the relevant edible oil standards in Kenya or east Africa to facilitate its control. This implies that there are chances that the consumers are exposed to unregulated levels of 3-MCPD despite the adverse health effects. This study sought to establish the levels of 3-MCPD esters in refined oils in the Kenyan market. The mean levels were compared with the minimum limit in the European Food Safety Authority (EFSA) of 0.8 µg/kg of the body weight. The study found that most of the refined oils in the country have significant levels of 3-MCPD esters when compared with the EFSA limits.
Keywords: Edible cooking oils, 3-Monochloropropane-1,2-diol (3-MCPD) esters, Carcinogenic
Cite this paper: Phyllyis Kiende, Alex King’ori Machocho, Muriira Geoffrey Karau, Investigation of the 3-Monochloropropane-1,2-Diol Levels in Edible Cooking Fats and Oils. A Case Study of the Kenyan Market, American Journal of Chemistry, Vol. 13 No. 4, 2023, pp. 102-106. doi: 10.5923/j.chemistry.20231304.02.
Article Outline
1. Introduction
- Most of the foods consumed by human beings contain fats and oils either processed or not. Apart from satiety, food has other symbolic meaning associated with dominion, affection, solace, security, stress reliever and honor [1]. Food contains nutrients which are classified as carbohydrates, dietary fibre, proteins, vitamins, water, minerals and lipids. Lipids can be solid called fats or liquid called oils. They can be of vegetable or animal origin and most of them are refined. They are triglyceride molecules which are esters made up of a glycerol backbone linked to three fatty acid molecules [2,3]. Oils are made up of large quantities of unsaturated fatty acids containing at least one double bond in the fatty acid chain. Dietary fats include trans-fats, monounsaturated, polyunsaturated and saturated fats. The structure of saturated fat is made up of single bond between carbon atoms throughout the fatty acid chain [4,5,6]. Polyunsaturated fats have multiples of double bonds between carbon atoms [7]. Refined edible oils are liquid at room temperature and when chilled turns to solid while fats are solids at room temperature [8,9].Fats and oils are refined chemically or physically before use. The process of the chemical purification of refined fats and oils is aimed at improving the final consumer characteristics such as clear appearance, stability to oxidation, light color, taste and aroma [10]. Further, refining process is aimed at achieving the required regulatory quality and safety levels. Chemical refining involves processes such as degumming, neutralization, bleaching and deodorization. The degumming process is the removal of phospholipids while neutralization is the removal of free fatty acids by addition of hydroxide solution which results in the formation of soap as a by-product [11].To remove unwanted odors and bittering agents from the crude oil to obtain a brand and odorless oil, deodorization step is carried out. This step involves distillation of the crude oils with steam at high conditions of temperatures of between 180-270°C, high vacuum and low pressure of 1.5-6.0 mbar to get rid of volatile compounds, free fatty acids and colors. In some cases, this process leads to the formation of 3-Monochloropropane-1,2-diol (3-MCPD) esters [12]. The 3-MCPD esters are believed to have been converted from triacylglycerol to cyclic acyloxonium in presence of chloride ions [13,14].Several studies have shown that 3-MCPD esters are carcinogenic and therefore a potential health concern [15,16]. The control Panel of European Food Safety Authority [16] investigated the effects of 3-MCPD on rats. Toxicity of the kidney was the significant effect [17]. Based on these results, a safe limit was set for human lifespan, maximum tolerable daily intake (MTDI) of 0.8 micrograms per kilogram of body weight daily (μg/kg body weight daily) [18]. The set limit for 3-MCPD by the joint Food Standards Australia and New Zealand (FSANZ) is 0.02 μg/kg body weight daily which is in line with EFSA standards. Joint committee of Expert on Food and Additives, FAO/WHO limit is 2 µg/kg body weight daily [19,20,21].Kenyans consume diverse products of food containing edible cooking fats and oils. No data shows existence or levels of 3-MCPD in edible cooking fats and oils. Additionally, there are no published regulatory limits in the regional or Kenyan Standards to facilitate control.
2. Materials and Methods
- Sampling and sample sizeThe fats and oils were sampled from various retail outlets in the Kenyan market. This study employed convenience method of sampling. A total of 57 brands of edible cooking fats and oils were recorded in Kenyan market during sampling period. Some of the samples were imported and others were locally manufactured. In this study, 30 samples of edible cooking fats and oils were sampled in triplicates. Analysis of the compound was done using American Oil Chemists society method [22].Sample preparationA portion of 250mg of edible fats and oils was weighed and put in 5ul centrifuge tubes and mixed with 1000ul of acetonitrile. The mixture was vortexed for 5minutes and centrifuged until a clear acetonitrile layer was obtained. 400ul of the acetonitrile layer was then transferred to a clean 2ul centrifuge tube and the solvent was removed under nitrogen stream at room temperature. The residue was reconstituted in 1ul of hexane and stored at -18°C before analysis.Isolation of 3-MCPD estersThe isolation of 3-MCPD esters was performed on a SunFire™C18 column (4.6 by 150 mm), 5um particle, using a Waters 1525 HPLC system with Waters 2424 evaporative light scattering detector (ELSD). The column temperature was maintained at 30°C, the sample injection volume was 20ul and each sample was injected three times. The mobile phase was acetonitrile (solvent A) and isopropanol (solvent B). The isocratic elution was performed as: 51% A, 49% B. The flow rate was 1ul min-1and both of the eluents containing 3-MCPD esters were collected and combined. The solvent was removed under nitrogen stream at room temperature. The sample was then reconstituted in 100ul acetonitrile and stored at -18°C before analysis.Determination of 3-MCPDestersThe 3-MCPD was determined using High-Performance Liquid Chromatography (HPLC) fitted with Evaporative Light Scattering Detector (ELSD). The column temperature during analysis was maintained at 30°C and the sample injection volume was 5ul. The mobile phase was composed of hexane (solvent A) and isopropanol (solvent B). The isocratic elution was performed as follows: 90% A, 10% B at a flow rate of 0.5ul min-1.The identity of 3-MCPD esters was verified by Ultra-high performance Liquid Chromatography with Quadrupole Time-Of-Flight Mass Spectrometry (UPLC-Q-TOF MS). The contents of 3-MCPD esters in oil samples were determined by HPLC method after the above described procedures of sample preparation and isolation of 3-MCPD esters. The concentration of 3-MCPD esters was calculated as 3-MCPD di-esters (1,2-dioleoyl-3-chloropropanediol standard substance) and 3-MCPD monoesters (1-stearoyl- 3-chloropropanediol standard substance). The quantitative analysis was based on the peak area of the retention time of 5.8 and 7.6 min, respectively. The calibration curve was constructed using the total 3-MCPD esters.
3. Results and Discussions
- The relationship between the logarithm of the peak area and the logarithm of the corresponding concentration of the standard substances was determined. The working range for the calibration solutions was 0.00 to 50.0 mg/kg and the linearity (R2) of 3-MCPD esters was 0.9981 as shown in Figure 1.
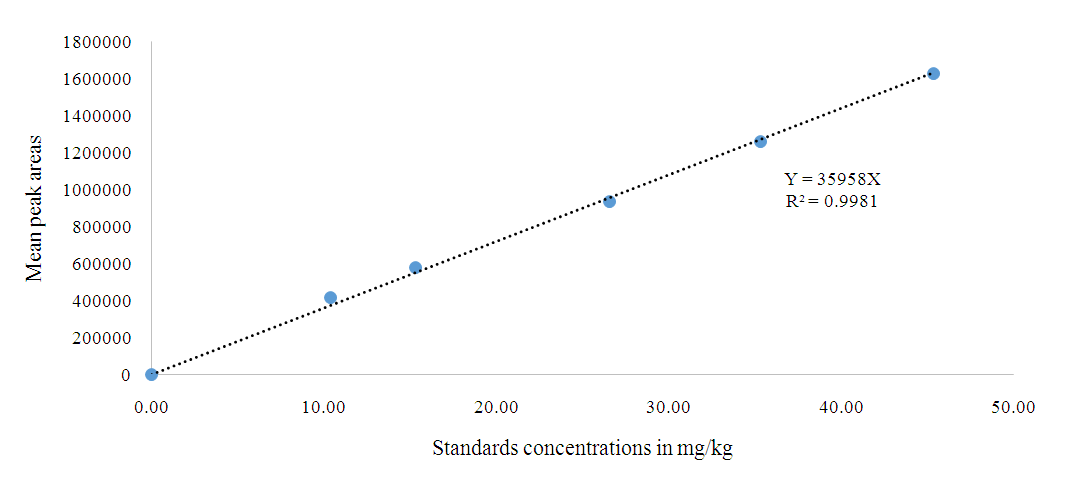 | Figure 1. Calibration curve of the concentration of 3-chloropropane-1, 2- diol in n-hexane in mg/kg against mean peak areas |
|
4. Conclusions
- This study reports high concentrations of 3-MCPD esters in refined edible cooking fats and oils in the Kenyan Market. The levels are above the regulatory limits set by the EFSA and other agencies. There are no regulatory limits for the Kenyan and East African regional market on the levels of consumption of 3-MCPD.
Declaration of the Conflict of Interest
- The authors declare no conflict of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML