-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2023; 13(4): 97-101
doi:10.5923/j.chemistry.20231304.01
Received: Aug. 20, 2023; Accepted: Aug. 29, 2023; Published: Aug. 31, 2023

A Simple and Rapid HPLC Method for Assay of Salbutamol, Ciprofloxacin, and Mannitol in a Triple Combination Dry Powder Formulation
Sunita Sule1, Sudesh Prabhakar Bhure2, Ameet Sule3, Sushama Raju Ambadekar1
1Department of Chemistry, Institute of Science, Madam Cama Road, Fort, Mumbai, India
2Independent Consultant, India
3H&T Presspart, UK
Correspondence to: Sunita Sule, Department of Chemistry, Institute of Science, Madam Cama Road, Fort, Mumbai, India.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

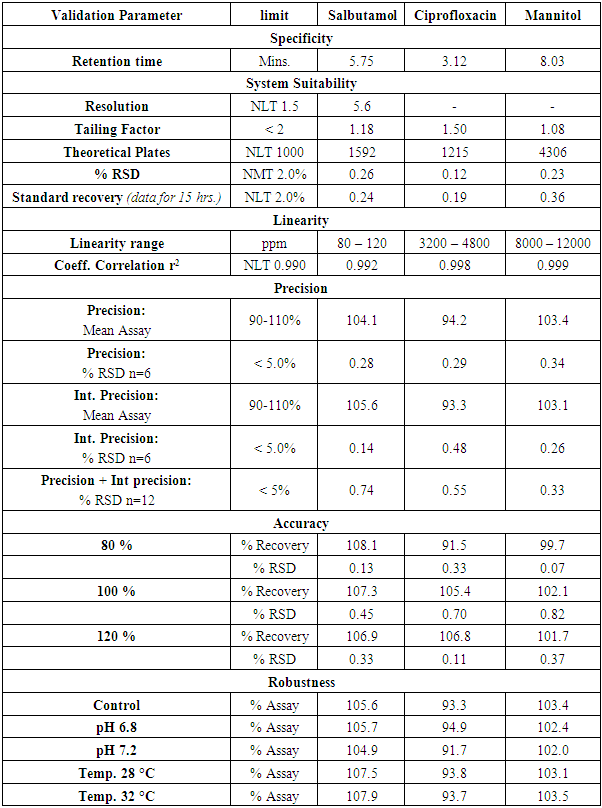
A simple, rapid, and novel high-performance liquid chromatography method has been developed and validated for simultaneous estimation of Salbutamol and Ciprofloxacin and Mannitol in a dry powder formulation for inhalation. The chromatographic separation was achieved using a Shodex NH2P-50 4D column and mobile phase composition of Buffer: Acetonitrile (20:80). The detection for Salbutamol and Ciprofloxacin was carried out in a UV detector at 225 nm, and Mannitol was detected in Refractive Index Detector. The method was validated in line with ICH guidelines and was found to be specific, linear, precise, accurate and robust. The % recovery was between 90-110 % for the assay, and the co-eff of linearity was greater than 0.990 for Salbutamol, Ciprofloxacin, and Mannitol. The % RSD for Inter-day and Intra-day Precision was less than 2%. This method will be useful in the assay of the novel combination product.
Keywords: Salbutamol, Ciprofloxacin, Mannitol, Validation, ICH guidelines
Cite this paper: Sunita Sule, Sudesh Prabhakar Bhure, Ameet Sule, Sushama Raju Ambadekar, A Simple and Rapid HPLC Method for Assay of Salbutamol, Ciprofloxacin, and Mannitol in a Triple Combination Dry Powder Formulation, American Journal of Chemistry, Vol. 13 No. 4, 2023, pp. 97-101. doi: 10.5923/j.chemistry.20231304.01.
Article Outline
1. Introduction
- Salbutamol Sulphate is a beta-2 adrenergic receptor agonist. It provides rapid and short-acting bronchodilation in reversible airway obstruction. It is used to treat bronchospasm caused by Asthma, COPD, and other lung diseases. In the respiratory tract, its effect is in the form of bronchial smooth muscle relaxation [1]. Ciprofloxacin belongs to the class of drugs called fluoroquinolone antibiotics. It is used to treat several bacterial infections, e.g., respiratory tract infections and urinary tract infections, among others. Its mode of action is by inhibiting enzymes topisomerase 11 and topoisomerase iv required for bacterial DNA replication [2]. Mannitol is a naturally occurring sugar that helps rehydrate mucus and airway clearances due to its osmotic properties [3]. The three drugs given in sequence are possible therapy routes in case of lung infections. [4]. The product under study is a novel dry powder for inhalation developed as a combination consisting of 0.2 mg Salbutamol (as Sulphate), 32.5 mg Ciprofloxacin (as Hydrochloride monohydrate) and 80 mg Mannitol via oral inhalation. Several analytical methods are available for the determination of Salbutamol Sulphate drug and drug product [5-9], Ciprofloxacin HCL and its drug products [10-14], Mannitol and its impurities [15-17]. However, none are reported for a combination drug product consisting of Salbutamol, Ciprofloxacin, and Mannitol. This paper focuses on developing a novel HPLC method for the simultaneous estimation of Salbutamol, Ciprofloxacin, and Mannitol in the combination product. The current study aimed to develop a simple, isocratic HPLC method with a suitable run time of less than 15 mins. The validation would be conducted in line with the current ICH guidelines.
2. Materials and Methods
- Reagents and chemicalsAcetonitrile HPLC-grade Finar, Phosphoric acid AR grade were procured from Finar (India). Triethylamine AR grade was purchased from Emparta (India).InstrumentationA High-Performance Liquid Chromatographic Shimadzu LC 20A system, equipped with an autosampler SIL 10 AC HT, column oven CTD-10AS VP, a UV detector SPD 20 A and a RID detector RID 20A was used for the analysis. The data was recorded using Lab Solutions software. Preparation of Mobile Phase Mobile phase: Buffer: ACN, 20:80Prepare a 0.15% orthophosphoric acid solution and adjust the pH to 7 with Triethylamine.Preparation of standard solution.Standard stock solution – A: About 12 mg of Salbutamol sulphate, about 469 mg of Ciprofloxacin Hydrochloride and about 1000 mg of mannitol were weighed in a 50 ml flask and dissolved in 30 ml water diluent by sonication and made up to the mark. (Stock Solution)Standard Solution - Assay: 5.0 ml of stock solution was transferred to a 10 ml volumetric flask was made up to the mark to give a solution containing 100 µl/ml of Salbutamol, 4000 µl/ml of Ciprofloxacin and 10000 µl/ml of mannitol. Preparation of Sample solutionSample solution 1: About 590 mg of sample blend of the novel DPI formulation was transferred in a 10 ml volumetric flask, dissolved, and diluted up to the mark to give a solution containing 100 µg/ml of Salbutamol, 16250 ppm Ciprofloxacin and 40,000 ppm of Mannitol.Sample Solution 2: 5 ml of the above solution was transferred in a 20 ml volumetric flask and diluted up to mark to give 4062.5 µl/ml solution of Ciprofloxacin and 10,000 µl/ml solution of Mannitol.Chromatographic condition
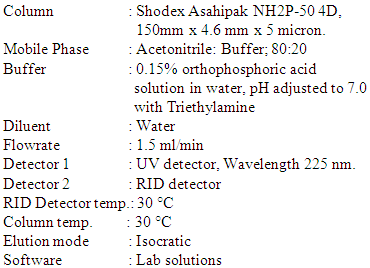 Method ValidationThe method was validated in line with the ICH guideline [20-21] for the test of assay, demonstrating the method is suitable for the intended use. Due to the high difference between the concentration of Salbutamol and Ciprofloxacin, the sample solution had to be prepared in two dilutions. The first dilution was used to estimate Salbutamol (100 ppm), and the second dilution was done to estimate Ciprofloxacin (4000 ppm) and Mannitol (10000 ppm).SpecificityThe specificity was performed by injecting solutions of individual Salbutamol sulphate, Ciprofloxacin HCl, Mannitol, and their mixed solution. Linearity Linearity test for the assay was performed on five concentration levels between 80 and 120% of the test concentration. Three replicates were injected for 80%, 90%, 100%, 110% and 120% concentration levels. Accuracy As there is no excipient in the formulation, the sample accuracy of the assay method was evaluated by determining the % recovery of Salbutamol. Ciprofloxacin and Mannitol at 80%, 100% and 120% of the test concentration. Precision System precision was performed by injecting and analyzing five replicate injections of Salbutamol, Ciprofloxacin, and Mannitol standard solution at the test concentration of Assay preparation. Method precision was performed by analyzing six individual sample preparation at 100% of the test concentration level of the Assay preparation. Intermediate precision was performed by testing six individual samples at 100% of the test concentration of Assay preparation on another day. Robustness The robustness of the proposed method was determined by studying the effect of small, deliberate changes in temperature (± 2°C) and change in pH (± 0.2) on the assay of the formulation.
Method ValidationThe method was validated in line with the ICH guideline [20-21] for the test of assay, demonstrating the method is suitable for the intended use. Due to the high difference between the concentration of Salbutamol and Ciprofloxacin, the sample solution had to be prepared in two dilutions. The first dilution was used to estimate Salbutamol (100 ppm), and the second dilution was done to estimate Ciprofloxacin (4000 ppm) and Mannitol (10000 ppm).SpecificityThe specificity was performed by injecting solutions of individual Salbutamol sulphate, Ciprofloxacin HCl, Mannitol, and their mixed solution. Linearity Linearity test for the assay was performed on five concentration levels between 80 and 120% of the test concentration. Three replicates were injected for 80%, 90%, 100%, 110% and 120% concentration levels. Accuracy As there is no excipient in the formulation, the sample accuracy of the assay method was evaluated by determining the % recovery of Salbutamol. Ciprofloxacin and Mannitol at 80%, 100% and 120% of the test concentration. Precision System precision was performed by injecting and analyzing five replicate injections of Salbutamol, Ciprofloxacin, and Mannitol standard solution at the test concentration of Assay preparation. Method precision was performed by analyzing six individual sample preparation at 100% of the test concentration level of the Assay preparation. Intermediate precision was performed by testing six individual samples at 100% of the test concentration of Assay preparation on another day. Robustness The robustness of the proposed method was determined by studying the effect of small, deliberate changes in temperature (± 2°C) and change in pH (± 0.2) on the assay of the formulation. 3. Results and Discussion
- Method OptimizationDevelopment studies were carried out by evaluating parameters influencing separation in chromatography. Salbutamol and Ciprofloxacin are ionic molecules, and Mannitol is a polar compound with no chromophore, thus not suitable for UV detection. The literature search revealed several RP HPLC methods for determining Salbutamol [4-8] and Ciprofloxacin [9-13] individually or with other drug substances using the C8 column and C18. Similarly, there are several methods for determining Mannitol, most of them using RI detector [15-19]. Initially, HPLC column configurations such as C8 And C 18 columns with 3-micron and 5-micron size particles in combination with a USP L19 column (to retain mannitol) in series were evaluated. The limitation was that the mobile phase could only consist of water and acetonitrile; a separation was achieved with the C18 and L19 columns in series. At lower concentration, separation of Salbutamol and Ciprofloxacin was achieved probably due to interactions with the stationary phase and that of Mannitol by ion exchange mechanism. However, due to the high concentration of Ciprofloxacin in the sample, the Salbutamol peak merged into the Ciprofloxacin peak. Further, the limitation of not being able to use a buffer for ionic molecules was an issue for getting good peak shape and repeatability for Salbutamol and Ciprofloxacin. In addition, there was a risk that the high concentration of the drugs would be detrimental to the L19 column packing. The target was to arrive at a column configuration that could support the buffered mobile required for the ionic Salbutamol and Ciprofloxacin as well as separate polar Mannitol. An alternate approach was to evaluate a Hydrophilic interaction liquid chromatography (HILIC) separation mode. HILIC mode consists of a polar stationary phase and a mobile phase consisting of a high percentage of the organic phase and a low percentage of the aqueous phase. A systematic approach on HILIC column configuration with a range of pH was evaluated, and the best separation was achieved with Shodex Asahipak NH2P – 50 4D column a 0.15% solution of Orthophosphoric acid with pH adjusted to 7 with triethyl amine. The stationary phase consists of spherical porous particles of polyvinyl alcohol modified with an amino group. Due to its polymer base, there would be reduced tailing due to no silanol activity [22]. Salbutamol and Ciprofloxacin are separated by anion exchange and HILIC mechanism and sugars by HILIC mechanism. To ensure salbutamol detection, which had a very low concentration, Vs Ciprofloxacin and mannitol sample solution was prepared as two dilutions, first for Salbutamol at 100 ppm and second for Ciprofloxacin at 4000 ppm and Mannitol 10000 ppm. To ensure the complete dissolution of the sample, the diluent selected was water which was different than the mobile phase and gave negative peaks in the RI detector at Ciprofloxacin retention time; hence Salbutamol and Ciprofloxacin were detected on the UV detector and Mannitol on the RID detector. The method was finalized after proper selection and optimization of the pH, sample solution concentration and ratio of Buffer and Acetonitrile. Separation was achieved for Salbutamol, Ciprofloxacin, and Mannitol in an isocratic mode and a run time of less than 15 mins Fig. 1 and 2.
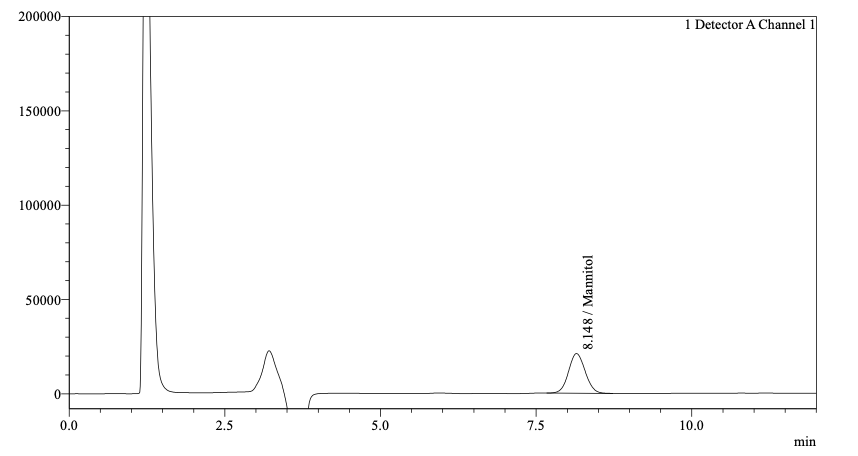 | Figure 1. Mannitol RT: 8.1 min in RID detector |
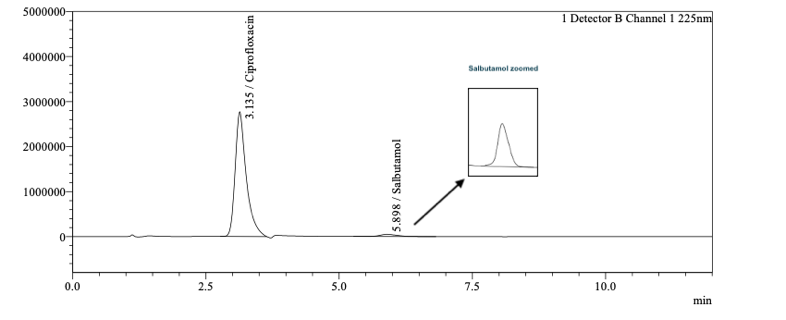 | Figure 2. Ciprofloxacin RT: 3.1 min Salbutamol RT: 5.9 mins in UV detector |
|
4. Conclusions
- A simple, economical, and rapid isocratic HPLC method has been developed and validated for quantitatively estimating Salbutamol, Ciprofloxacin and Mannitol on a single HPLC column and two detectors (UV and RID). The HPLC method was validated in line with ICH guidelines. The results show that the developed method is sensitive, linear, precise, accurate and robust. The developed method can be used for quantitative estimation of Salbutamol, Ciprofloxacin and Mannitol, making it suitable for QC release and stability studies.
ACKNOWLEDGEMENTS
- We express our gratitude to Mr Nilesh Dhamorikar and Qbd Labs for their support in conducting this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML