-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2023; 13(3): 83-95
doi:10.5923/j.chemistry.20231303.03
Received: Jul. 26, 2023; Accepted: Aug. 17, 2023; Published: Aug. 28, 2023

Synthesis, Characterization and In-Vitro Antibacterial Propriety of Cu(II), Co(II) and Bi(III) Complexes with (Z)-1-(2-Phenylhydrazono) Naphthalen-2(1H)-one as a Ligand
Emmanuel F. Sopbué1, Raoul K. Dennue1, Jean-de-Dieu Tamokou2, Apollinaire Tsopmo3, Giscard Doungmo4, Peter F. W. Simon5, Bruno N. Lenta6, Jules R. Kuiate2
1Laboratory of Applied Synthetic Organic Chemistry, Department of Chemistry, Faculty of Science, University of Dschang, Dschang, Republic of Cameroon
2Research Unit of Microbiology and Antimicrobial Substances, Department of Biochemistry, Faculty of Science, University of Dschang, Dschang, Republic of Cameroon
3Department of Chemistry, Carleton University, Colonel By Drive K1S 5B6, Ottawa, Canada
4Institut für Anorganische Chemie, Christian-Albrechts-Universität zu Kiel, Max-Eyth-Str. 2, 24118 Kiel, Germany
5Polymer Chemistry Laboratory, Faculty of Life Sciences, Rhine-Waal University of Applied Sciences, Campus Kleve, Marie-Curie Strasse 1, D-47533 Kleve, Germany
6Higher Teacher’s Training College, University of Yaounde I, Yaounde, Cameroon
Correspondence to: Emmanuel F. Sopbué, Laboratory of Applied Synthetic Organic Chemistry, Department of Chemistry, Faculty of Science, University of Dschang, Dschang, Republic of Cameroon.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The present work deals with the synthesis, characterization and antibacterial evaluation activity of new transition metals complexes obtained from the reaction of CoC2O4.2H2O, CuCl2.2H2O and BiCl3 with 1-(2-phenylhydrazono) naphthalen-2-one used as ligand. The structures of the ligand and its complexes were assigned on the basis of the available spectroscopic (IR, UV, 1&2D NMR, powder XRD), spectrometric (ESI-MS) and elemental analyses data. In-vitro antibacterial activity of the synthesized compounds have been screened against gram positive and gram-negative bacteria by using microdilution method. The antibacterial activity assay results showed that metal complexes (CMI = 32-128 μg/mL) possess higher antibacterial activity compared to the free ligand (CMI = 64-128 μg/mL).
Keywords: Synthesis, Hydrazone ligand, Chelate complexes, Antibacterial activity
Cite this paper: Emmanuel F. Sopbué, Raoul K. Dennue, Jean-de-Dieu Tamokou, Apollinaire Tsopmo, Giscard Doungmo, Peter F. W. Simon, Bruno N. Lenta, Jules R. Kuiate, Synthesis, Characterization and In-Vitro Antibacterial Propriety of Cu(II), Co(II) and Bi(III) Complexes with (Z)-1-(2-Phenylhydrazono) Naphthalen-2(1H)-one as a Ligand, American Journal of Chemistry, Vol. 13 No. 3, 2023, pp. 83-95. doi: 10.5923/j.chemistry.20231303.03.
Article Outline
1. Introduction
- During the last decade azo compounds are well known for their wide use in the dyeing of textile fibers and coloring of different materials, e. g. for plastics, biological as well as medical studies. Furthermore, they are also well established for advanced applications in organic synthesis and high technology areas such as laser, liquid crystalline displays, electro-optical devices and ink-jet printers [1,2]. Therefore, a large number of (N, O)-donor ligands in azo imine family have been prepared [3,4] and used to form very stable chelate complexes with various applications such as optical data storage [5], photo switching, nonlinear optics and photochromic materials, dyes, chemical analysis [6,7], and pharmaceuticals [8]. Both azo dyes and their metal complexes play an essential role in the chemistry of living organisms and their biological importance continues to be studied [9]. Although a rapid and convenient synthesis of azo dyes ligands and their metal complexes have already been described, the UV-visible spectral behavior in different pH regimes and temperatures as well as biological activities are yet to be studied [7,10,11]. However, during the synthesis process of azo compound, an intramolecular proton transfer between the azo nitrogen atom and hydroxyl oxygen atoms may result in a tautomer hydrazone structure, if both groups feature a para/ortho substitution pattern. This hydrazone tautomer has been studied using various techniques, including UV-VIS and NMR spectroscopy and computational simulations [9,12]. In fact, proton tautomerism plays an important role in many branches of chemistry, particularly biochemistry [9,13]. They can be used as a potential chelating ligand for the synthesis of metal complexes of different geometry which leading to a remarkable property profile and potential uses such like optical signal processing, molecular data processing, ion sensors and biological applications [4].According to the fact that, a metal complex sometimes exhibits better biological activity than the corresponding ligand, many researches focused their work on the synthesis of new metal complexes. To mention a few examples, a cadmium complex with 4-(2-pyridyl azo)-resorcinol, is used as an anti-tumor drug [11], whereas Co (II) and Cu (II) azo-naphthol chelate complexes give a good antibacterial activity [8,11,14]. These previous findings motivated us to embark on the synthesis, characterization of some transition metal complexes and the evaluation of the antibacterial activities of both the ligand and the metals complexes in organic solvents of different polarities, and at different temperatures. The coordination behavior of these hydrazone ligands towards copper (II), cobalt (II) and bismuth (III) ions will also be investigated using different analytical tools. Finally, the antimicrobial effects of these compounds on Gram-positive and Gram-negative bacteria will be explored.
2. Experimental Section
2.1. Material and General Manipulations
- All the reagents and solvents were purchased from Sigma-Aldrich and Fluka and they were used without further purification. The reaction end and the purity of the compounds was determined using thin layer chromatography (TLC plates coated silica gel 60 F254) in a mixture of solvent like ethyl acetate and hexane (10:90, v/v). Visualization was achieved either by UV light (254 nm) or iodine after elution. The melting points of synthetized compounds were determined using a STUART SCIENTIFIC Melting Point Apparatus Model SMP20. Elemental analysis was carried out with Euro EA 3000 from Hekatech GmbH, Friedrich-List-Allee 26, 41844 Wegberg. Infrared spectra were recorded on a Bruker Alpha spectrophotometer (FT-IR Bruker Optik GmbH, Rudolf-Plank-Str. 27, 76275 Ettlingen) using ATR (Attenuated Total Reflectance) technique on a diamond crystal. The UV-visible absorption spectra were recorded using a UNICAM UV 300 spectrophotometers with a quartz cell of an optical path length of 1 cm. The UV-spectra were recorded in the range from 200 to 1000 nm. The ESI positive spectra mass were recorded on a Compact BRUKER spectrometer with a DIONEX Ultimate 3000 brand LC chain. Nuclear magnetic resonance (NMR) experiments (1D and 2D) were performed in DMSO-d6 and MeOH-d4/CDCl3 on a 400 MHz JEOL ECZ spectrometer equipped with 5-mm digital auto tune Royal probe (JEOL USA, Peabody, MA). 1H-NMR spectral data were recorded at 400 MHz while 13C-NMR data were measured at 100 MHz both with TMS used as internal reference. Powder XRD data was collected on a STOE Stadi-p X-ray powder diffractometer (STOE & Cie GmbH, Darmstadt, Germany) with Cu Kα1 radiation (λ = 1.54056 Å; Ge monochromator; flat samples) in transmission geometry with a DECTRIS® MYTHEN 1K detector (DECTRIS, Baden-Daettwil, Switzerland). Elemental analyses were performed with a Euro Vector CHNS-O element analyzer (Euro EA 3000) or a vario MICRO Cube (Co. Elementa Analyzer system). Theorical calculations were performed with Gaussian 9 software in a B3LYP/6-311G mode. Sonochemical reaction was carried out in an ultrasonic vessel (45 kHz, 80 W). The antibacterial activities were performed using the microdilution method and then the minimal concentration inhibition was determined to evaluate the activity of compounds studied.
2.2. Synthesis of (Z)-1-(2-Phenylhydrazono) Naphthalen-2(1H)-one (5)
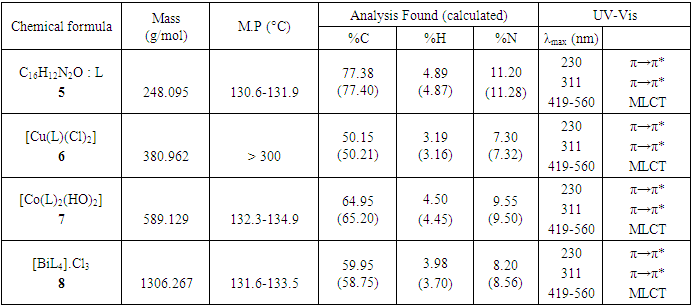
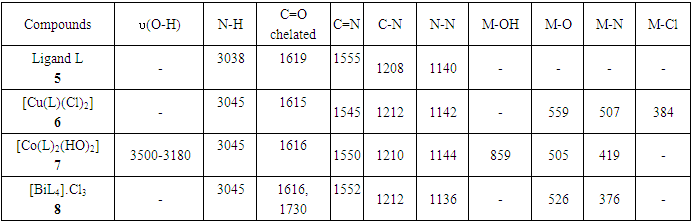
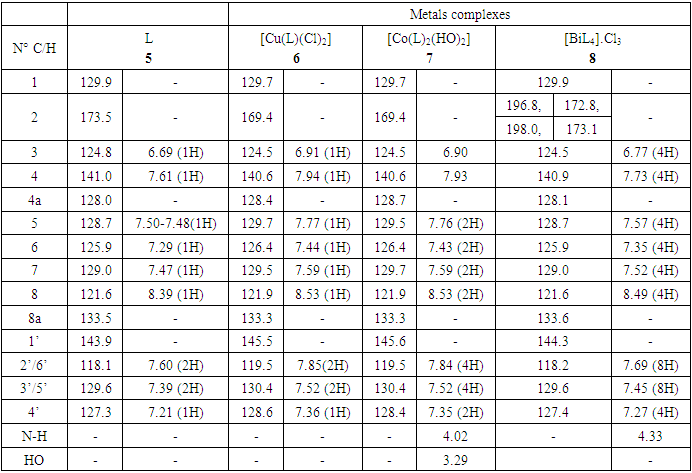
- The azo-naphthol tautomer ligand (L) with the structure depicted in scheme 1 was prepared using the known coupling methods [8,11]. In particular, DMSO-solutions of aniline (1.82 g, i. e. 20 mmol in 5 ml) and of sodium nitrite (1.5 g, i. e. 21.5 mmol in 7 ml) were prepared. Both solutions were thoroughly mixed in an Erlenmeyer flask and placed into a salted ice bath to achieve a temperature range between 0 and 5°C. Under continuous stirring 1.7 ml of concentrated hydrochloric acid is added in small portions; to complete the formation of the diazonium salt stirring continues for about 1 hour. Subsequently 2.88 g (20 mmol) of β-naphthol are dissolved in a 10% sodium hydroxide solution and then gradually added to the cooled aniline diazonium chloride salt. The resulting mixture was continually stirred at 0–5°C for 2 h to complete the reaction. The reaction medium is poured into 250 ml of ice water until the formation of a precipitate can be observed. The precipitate is recovered by filtration and washed with cold and hot water and further purified by recrystallization with ethanol. This procedure leads to a product which, when dried at ambient temperature, gives 3.17 g (yield 75%, m.p. 130.6-131.9°C) of a dark red powder; IR (ATR) νmax (cm-1): 3038 (N-H----O), 1619 (C=O---H), 1597 (C=C), 1555 (C=N), 1208 (C-N), 1140 (N-N); UV-visible (ethanol) λmax (nm) 483-560 (CT), 421, 311 (π→π*); 230 (π→π*); 1H-NMR (methanol-d4/CDCl3, 400 MHz) δH: 8.39 (1H, d, J = 8 Hz, H-8), 7.61 (1H, d, J = 9.6 Hz, H-4), 7.60 (2H, d, J = 8.4 Hz, H-2’/H-6’), 7.50-7.48 (1H, m; H-5), 7.47 (1H, m, H-7), 7.39 (2H, m, H-3’/H-5’), 7.29 (1H, m, H-6), 7.21 (1H, m, H-4’), 6.69 (1H, d, J = 9.6 Hz, H-3), estimated 16 ppm (NH, not experimentally accessible); 13C-NMR (methanol-d4/CDCl3, 100 MHz) δC: 173.5 (1C, C-2), 143.9 (1C, C-1’), 141.0 (1C, C-4), 133.5 (1C, C-8a), 129.9 (1C, C-1), 129.6 (2C, C-3’/C-5’), 129.0 (1C, C-7), 128.7 (1C, C-5), 128.0 (1C, C-4a), 127.3 (1C, C-4’), 125.9 (1C, C-6), 124.8 (1C, C-3), 121.6 (1C, C-8), 118.1 (2C, C-2’/C-6’). (HRESI+): protonated molecular ion [M+H].+ m/z 249.1024. Elemental analysis: C16H12N2O Found (calculated): C: 77.38 (77.40); H: 4.89 (4.87); N: 11.20 (11.28).
2.3. Synthesis of Metal Complexes
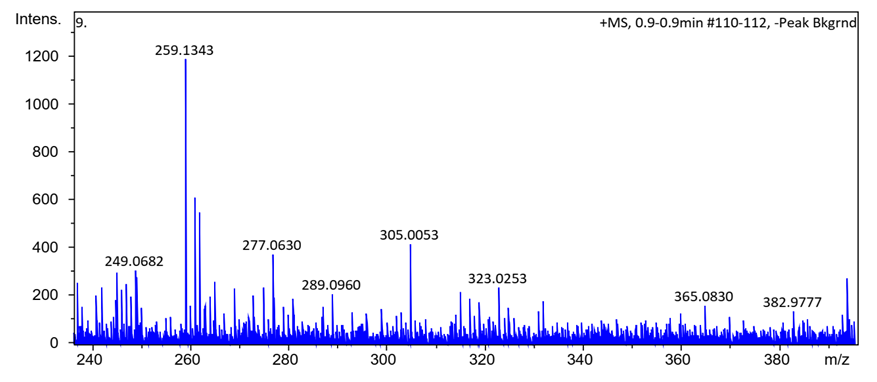
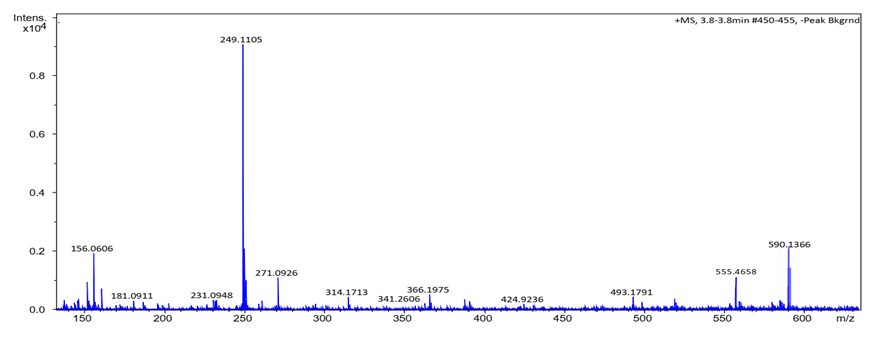
- Synthesis of Copper (II) Complex 6: 0.25 g (1 mmol) of ligand L (5) was heated with copper chloride (CuCl2.2H2O) 0.1705 g (1 mmol) under reflux for 6 hours in 15 mL of acetone. After cooling to room temperature, the homogeneous mixture was poured into cold NaCl solution and then kept for 15 min in an ice bath. The crude precipitate was collected by filtration, washed several times with water and recrystallized in ethanol to give 0.27 g (70%, m.p > 300°C) of dark blue powder; IR (ATR) νmax (cm-1): 3045 (N-H----O), 1615 (C=O---H), 1595 (C=C), 1545 (C=N), 1212 (C-N), 1142 (N-N), 384 (Cu-Cl); 507 (Cu-N) et 559 (Cu-O); UV-visible (ethanol) λmax (nm), 483-560 (MLCT), 421, 311 (π→π*); 230 (π→π*); 1H-NMR (DMSO-d6, 400 MHz) δH: 8.53 (1H, d, J = 8 Hz, H-8), 7.94 (1H, d, J = 9.2 Hz, H-4), 7.85 (2H, d, J = 8 Hz, H-2’/H-6’), 7.77 (1H, d; J = 7.8 Hz; H-5), 7.59 (1H, m, H-7), 7.52 (2H, m, H-3’/H-5’), 7.44 (1H, m, H-6), 7.36 (1H, m, H-4’), 6.91 (1H, d, J = 9.2 Hz), estimated 16 ppm (NH, not experimentally accessible). 13C-NMR (DMSO-d6 100 MHz) δC: 169.4 (1C, C-2), 145.5 (1C, C-1’), 140.6 (1C, C-4), 133.3 (1C, C-8a), 129.7 (1C, C-1), 130.4 (2C, C-3’/C-5’), 129.5 (1C, C-7), 129.7 (1C; C-5), 128.4 (1C, C-4a), 128.6 (1C, C-4’), 126.4 (1C, C-6), 124.5 (1C, C-3), 121.9 (1C, C-8), 119.5 (2C, C-2’/C-6’). MSESI+: protonated molecular ion [M+2H].+ m/z 382.9777. Elemental analysis Cu(L)(Cl)2 Found (calculated) C: 50.15 (50.21); H:3.19 (3.16); N:7.30 (7.32). Synthesis of Cobalt (II) Complex 7: To a warm ethanol solution (10 mL) containing 0.25 g (1 mmol) of ligand 5, a mixture of 0.2 g (0.5 mmol) of cobalt oxalate dihydrate salt in 5 mL ethanol was gradually added. The resulting red mixture was subjected to ultrasonic stirring for 2 hours. The resulting solution was filtered and the filtrate was left to crystallize at room temperature for about 24 hours. The precipitate formed was collected by filtration, washed several times with ice water and a water/ethanol mixture (50:50; v/v), and dried at room temperature to afford 0.55 g (80%, m.p. 132.3-134.9°C) of compound 7 as brown powder; UV-visible (ethanol) λmax (nm) 483-560 (MLCT), 421, 311 (π→π*); 230 (π→π*); IR (ATR) νmax (cm-1): 3500-3180 (OH, H2O), 3045 (N-H), 1616 (C=O), 1597 (C=C), 1550 (C=N), 1144 (N-N), 859 (Co-OH), 505 (Co-O), 419 (Co-N); 1H-NMR (DMSO-d6, 400 MHz) δH : 8.53 (2H, d, J = 8 Hz; H-8), 7.93 (2H, d, J = 9.2 Hz, H-4), 7.84 (4H, d, J = 8 Hz, H-2’/H-6’), 7.76 (2H, d, J = 7.6 Hz, H-5), 7.59 (2H, m, H-7), 7.52 (4H, m, H-3’/H-5’), 7.43 (2H, m, H-6), 7.35 (2H, m, H-4’), 6.90 (2H, d, J = 9.2 Hz), 4.02 (s, NH), 3.29 (s, H2O); 13C-NMR (DMSO-d6 100 MHz) δC: 169.4 (2C, C-2), 145.6 (2C, C-1’), 140.6 (2C, C-4), 133.3 (2C, C-8a), 129.7 (2C, C-1), 130.4 (4C, C-3’/C-5’), 129.7 (1C, C-7), 129.5 (2C, C-5), 128.7 (2C, C-4a), 128.4 (2C, C-4’), 126.4 (2C; C-6), 124.5 (2C, C-3), 121.9 (2C, C-8), 119.5 (4C, C-2’/C-6’). MSESI+: [M+H]+ m/z 590.1366. Elemental analysis Co(L)2(OH)2 Found (calculated) C: 64.95 (65.20) H: 4.50 (4.45); N: 9.55 (9.50).Synthesis of Bismuth (III) Complex 8: Bismuth (III) complex 8 was synthesized in analogy to the method described above. A solution of 0.3 g (1 mmol) of BiCl3 in acetone (3 mL) was added dropwise to a mixture of 0.5 g (2 mmol) of ligand 5 in 5 mL of acetone to obtain 1.05 g (78%, m.p. 131.6-133.5°C) of a dark black powder; UV-visible (ethanol) λmax (nm) 483-560 (MLCT), 421, 311 (π→π*); 230 (π→π*); IR (ATR) νmax (cm-1): 3045 (N-H), 2920 (C-H), 2852 (Ar-H), 1730, 1616 (C=O), 1552 (C=N), 1212 (C-N) 1136 (N-N), 526 (Bi-O), 376 (Bi-N); 1H-NMR (methanol-d4/CDCl3, 400 MHz) δH: 8.49 (4H; d, J = 8 Hz; H-8), 7.73 (4H, d, J = 9.6 Hz, H-4), 7.69 (8H, d, J = 7.6 Hz, H-2’/H-6’), 7.57 (4H, m, H-5), 7.52 (4H, m, H-7), 7.45 (8H, m; H-3’/H-5’), 7.35 (4H, m, H-6), 7.27 (4H, m, H-4’), 6.77 (4H, d, J = 9.6 Hz, H-3), 4.33 (s, NH); 13C-NMR (methanol-d4/CDCl3, 100 MHz) δC: 196.8, 198.0, 172.8, 173.1 (4C; C-2), 144.3 (4C, C-1’), 140.9 (4C; C-4), 133.5 (4C, C-8a), 129.9 (4C, C-1), 129.6 (8C, C-3’/C-5’), 129.0 (4C, C-7), 128.7 (4C, C-5), 128.1 (4C, C-4a), 127.4 (4C, C-4’), 125.9 (4C, C-6), 124.5 (4C, C-3), 121.6 (4C, C-8), 118.2 (8C, C-2’/C-6’), MSESI+ [M-2Cl+Na].+ at m/z 1259.3121. Elemental analysis, [BiL4].(Cl)3 % Found (Calculated): C: 59.01 (58.75); H: 3.80 (3.70); N: 9.20 (9.32).
2.4. Biological Assay
2.4.1. Microorganisms
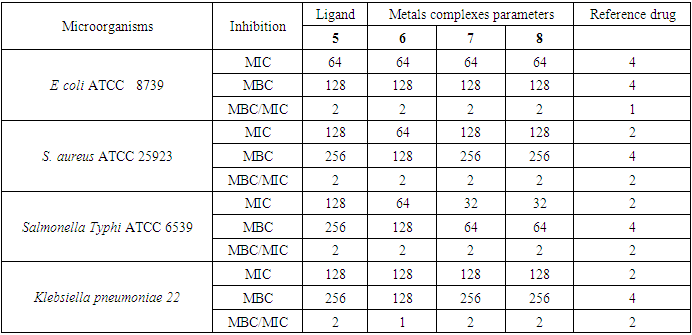
- The in-vitro antibacterial activity of ligand 5 (L) and their metal complexes (6, 7 and 8) was assayed against three gram-negative (Escherichia coli. ATCC 8739, Salmonella Typhi ATCC 6539, Klebsiella pneumoniae 22) and one Gram-positive (Staphylococcus aureus ATCC 25923) bacterial strains by the reported method [15]. All strains are reference ones obtained from the American Type Culture Collection. The bacterial strains were grown at 35°C and maintained on nutrient agar (NA, Conda, Madrid, Spain) and the Minimum inhibitory concentrations (MIC) were determined by the liquid micro-dilution method as described earlier [15,16].
2.4.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
- The minimum inhibitory concentration (MIC) was determined using the established method [16]. Micro titer plates (96 micro wells) were prepared and each well received 85 μl of Mueller Hinton Agar and 5 μl of inoculum. The ligand (5) and its metal complexes 6, 7, 8 were made at concentrations of 2 mg/mL in 5% (v/v) aqueous solution of dimethylsulfoxide (DMSO) at 5% (v/ v). One plate was used for each group of microorganisms. The positive control contained the appropriate medium and the microbial suspension only, whereas negative control was done with an aqueous solution of DMSO or 10% Tween 20 instead of the inoculum. 10 μl of each tested compound were prepared and added in subsequently to give a final volume of 100 μl. The plates were covered and incubated with shaking at 35°C for 24 hours. Microbial growth was determined by introducing 5 μl of a 2 mg/ml para-iodonitrotetrazolium solution. Any color change from yellow to purple indicates microbial growth. The minimum inhibitory concentration has been defined as the smallest concentration of an antibiotic or substance that prevents this color change. 10 μl of the contents of each well were withdrawn aseptically and spread separately on the surface of the medium of Muller Hinton Agar in order to determine the minimum bactericidal concentration (MBC) which is defined as being the smallest concentration giving a sub-negative culture or only one colony. Three replicates were performed for each sample; ciprofloxacin was used as reference drug.
3. Results and Discussion
3.1. Chemistry
- The reaction of (Z)-1-(2-phenylhydrazono) naphthalen-2(1H)-one ligand (L) with the metal ions Cu (II), Co (II), Bi (III), gave different colored powder, depending on the nature of metal ion. The metal complexes were air-stable, insoluble in water, but soluble in some common organic solvents. The synthesized ligand and their metals complexes were characterized by various analytical techniques such as spectroscopy (IR, 1D & 2D NMR, UV-Vis), ESI-mass spectrometric and elemental analysis. The synthesis reaction of the ligand and its metals complexes is depicted in schemes 1 and 2. Physical and analytical analysis are given in table 1, whereas the IR spectroscopy of a ligand and their metal complexes are given in table 2. 1H- & 13C-NMR data are show in table 3. The antibacterial activity of ligand and their metals complexes are listed in table 4.
|
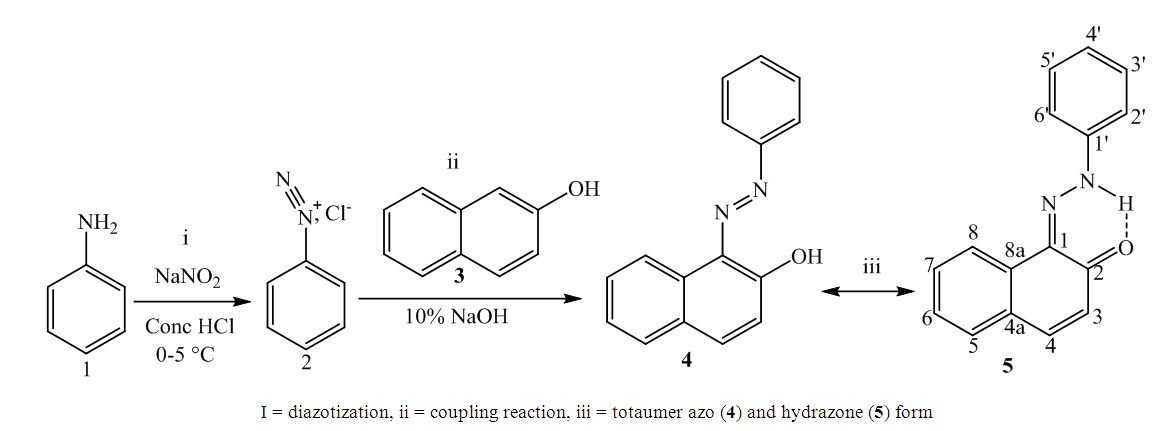 | Scheme 1. Reactions’ sequences to ligand 5 |
|
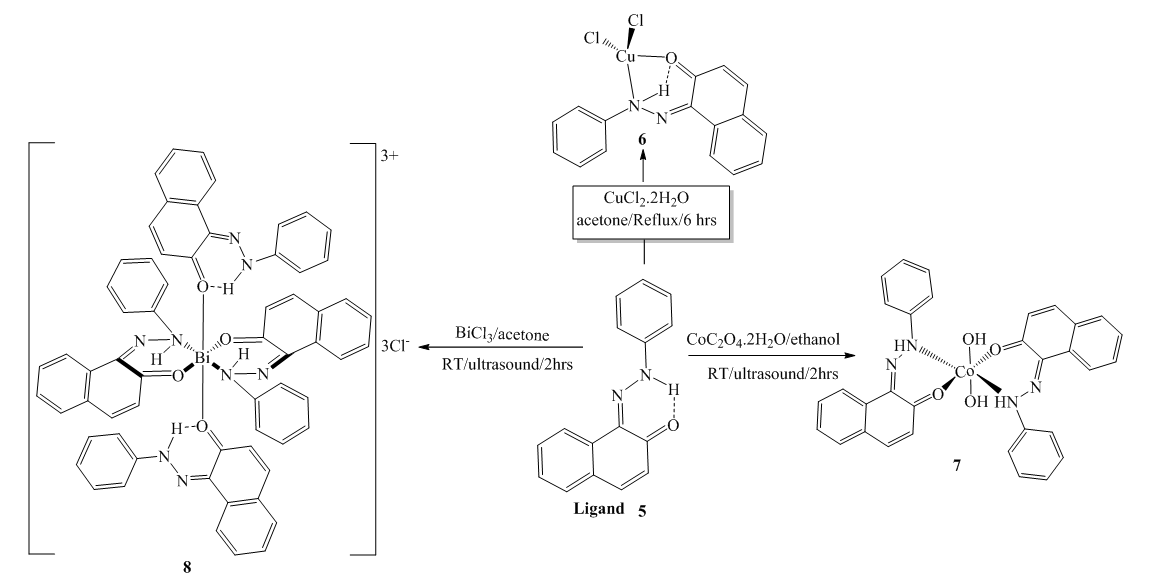 | Scheme 2. Reactions sequences to the metal complexes 6, 7 and 8 |
|
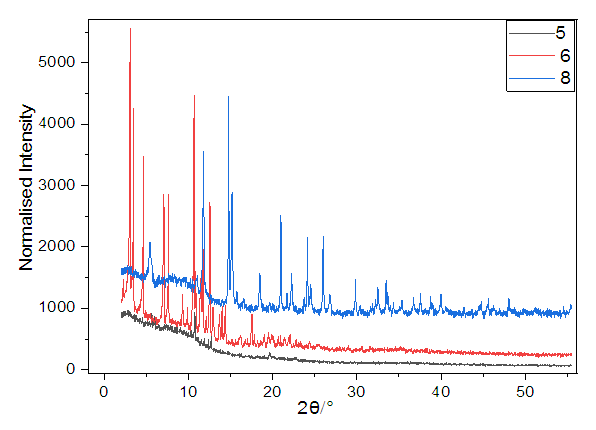 | Figure 1. XRPD Patterns of the ligand (5) and of complexes 6 and 8 |
3.2. Biology
- The antibacterial effects of ligand 5 and its metals complexes 6, 7 and 8 against gram-positive and gram-negative bacteria are compiled in table 4. All the compounds synthetized showed different degrees of antibacterial activity against tested bacterial (MIC = 32-128 μg/mL) but did not exceed the activity of the reference drug ciproflocine. Nevertheless, the metals complexes had either the same or a higher activity as the ligand. The copper (II) complex 6 showed an intermediated activity (MIC = 64 μg/mL) against E. coli, S. aureus, Salmonella Typhi and the least one (MIC = 128 μg/mL) against Klebsiella pneumoniae. The cobalt and bismuth complexes 7 and 8 had an intermediated activity (MIC = 64 μg/mL) against E. coli, and the least one (MIC = 128 μg/mL) against S. aureus and Klebsiella pneumoniae. However, both complexes are significantly more active against Salmonella Typhi (MIC = 32 μg/mL) than the free ligand. Based on MBC and MBC/MIC results in table 4, all metal complexes and ligand had a bacteriostic effect (MBC/MIC = 2) against the tested bacterial strains, except the copper complex which had an absolute bactericidal effect (MBC/MIC = 1) against Klebsiella pneumoniae. Presumably, the structural factors which govern antimicrobial activities are strongly dependent on the central metal ion [29]. The difference activity between ligand and their metals complexes are since described basis of overtone’s concept of cell permeability.
|
3.3. HOMO-LUMO Analysis and Molecular Electrostatic Potential (MEP)
- Computational methods are very important in chemistry as they allow a prediction of many chemical properties as well as polarizability, chemical reactivity and kinetic stability of the molecule synthetized. Therefore, the energy band gap (HOMO-LUMO) and the global reactivity descriptors of the ligand and it metals complexes are evaluated using hybrid functional B3LYP and triple zeta basis set with both diffuse functions, 6-311++G (d, p) [33]. The calculations indicate that the ligand 5 has 65 occupied molecular orbitals and the value of the energy separation between the LUMO and HOMO is 2.999 eV. Therefore, in order to identify the electrophilic and nucleophilic regions, Molecular Electrostatic Potential (MEP) was computed for an optimized structure and the results are given in figure 2. The red color specifies the higher negative potential regions which are beneficial for electrophilic attack, whereas the blue color identifies the higher positive potential regions favorable for nucleophilic attack.
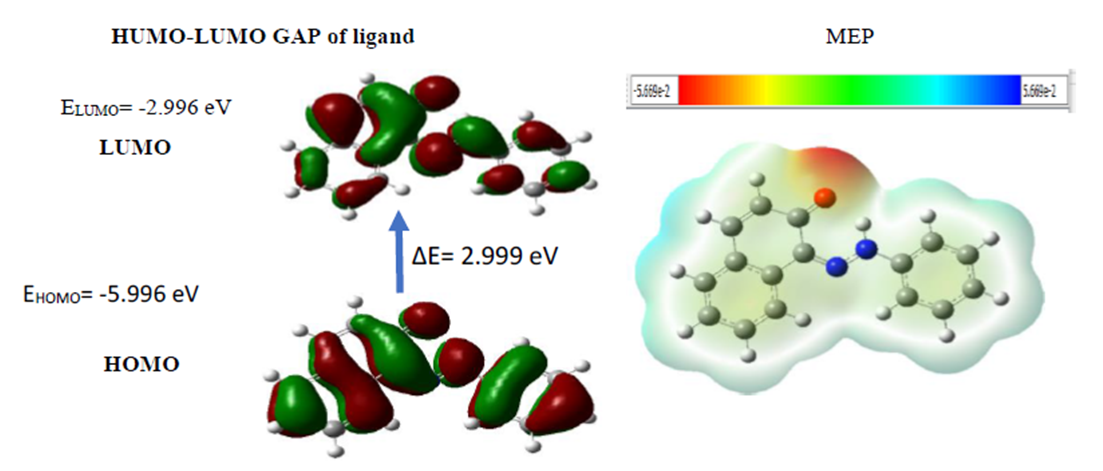 | Figure 2. Structures of the FMO (HOMO and LUMO) and MEP of Compound 5 |
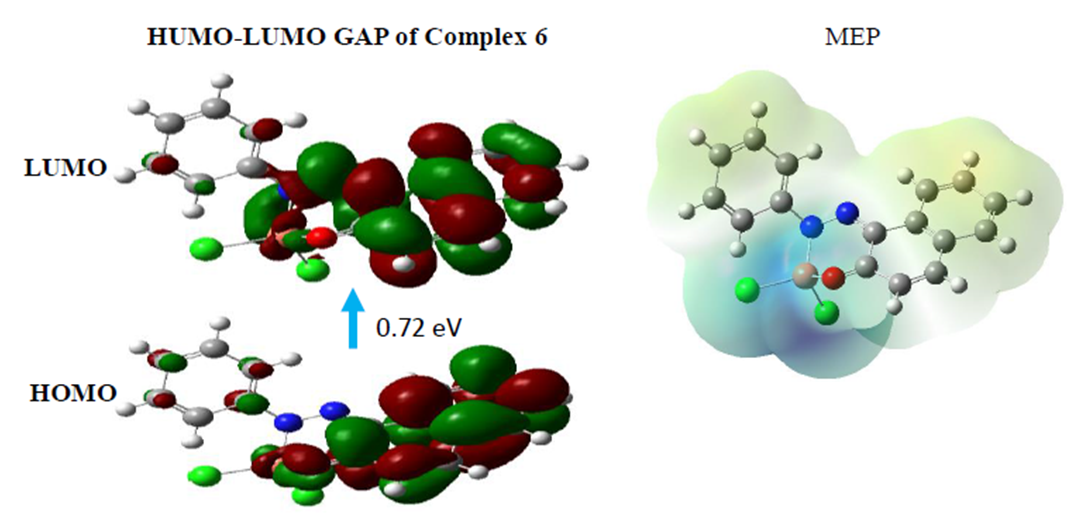 | Figure 3. Structures of the FMO (HOMO and LUMO) and MEP of compound 6 |
4. Mass Spectrum and Fragmentations’ Patterns of the Compounds
- HRESI+ mass spectrum of ligand and their metal complexes are presented in figure 4 (4a, 4b, 4c, 4d).
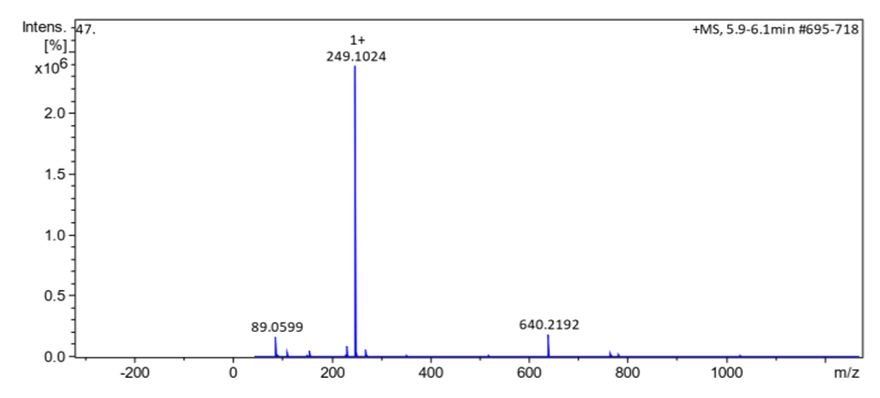 | Figure 4a. HRESI+ mass spectrum of ligand 5 |
 | Figure 4b. HRESI+ mass spectrum of complex 6 |
 | Figure 4c. HRESI+ mass spectrum of complex 7 |
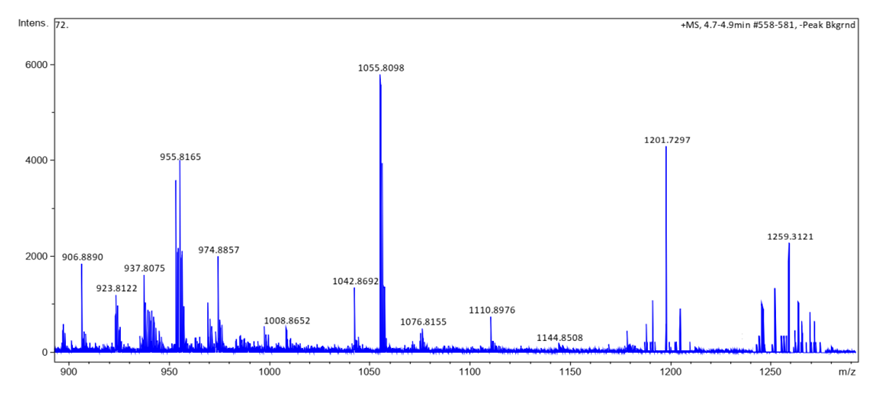 | Figure 4d. HRESI+ mass spectrum of complex 8 |
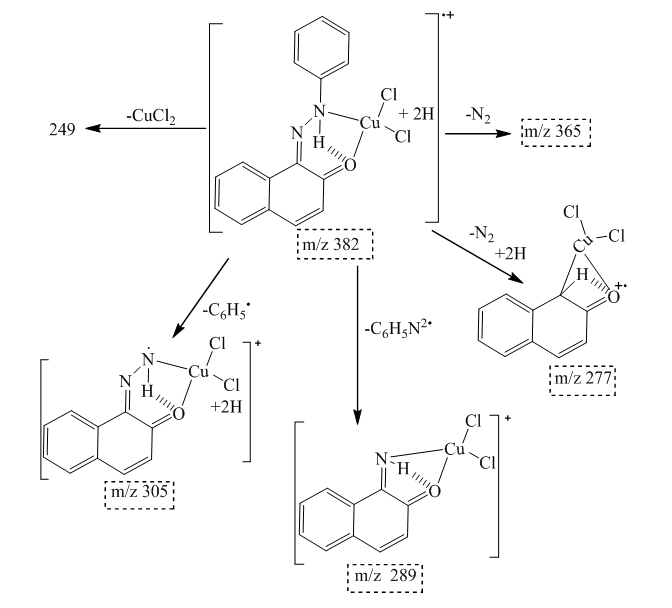 | Scheme 3. Fragmentation pattern of complex 6 |
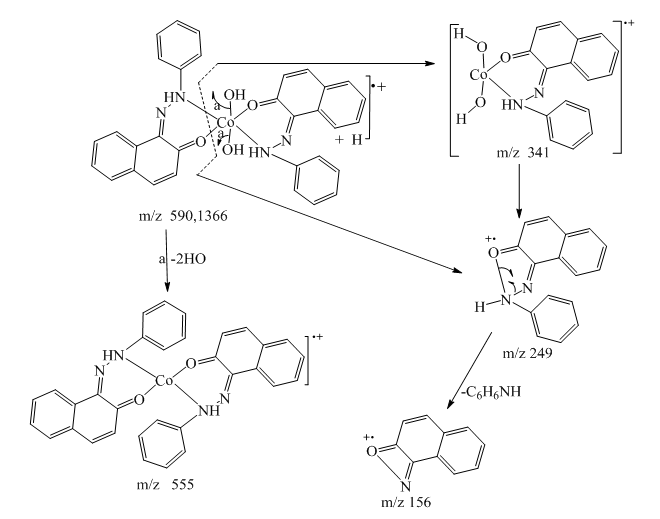 | Scheme 4. Fragmentation pattern of complex 7 |
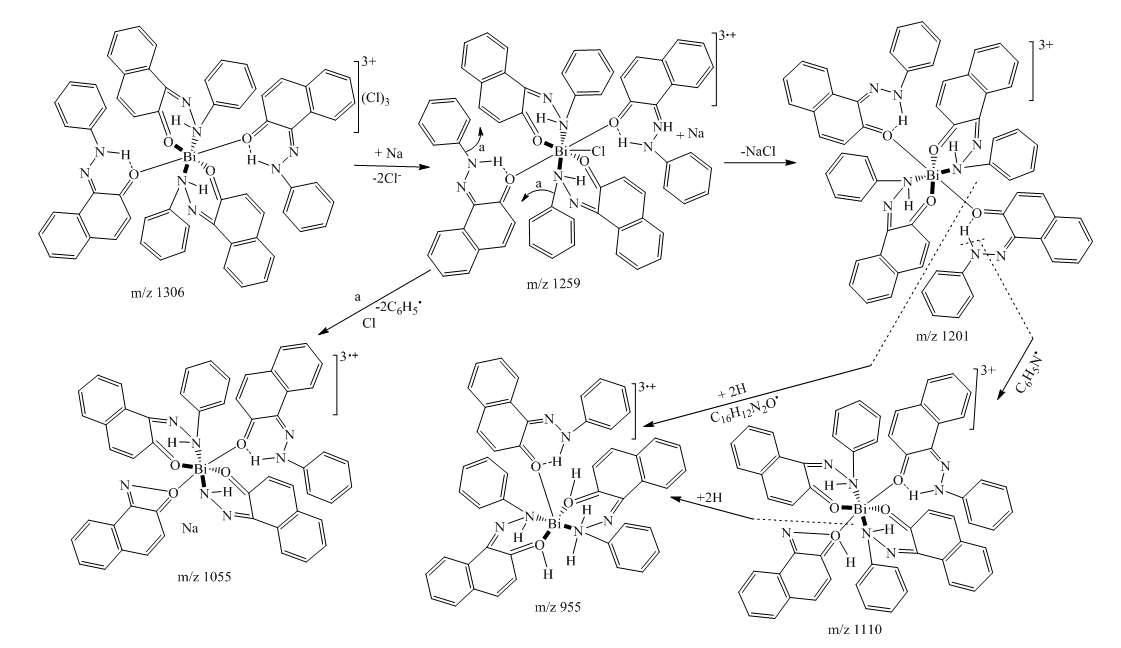 | Scheme 5. Fragmentation pattern of complex 8 |
5. Conclusions
- The synthesized ligand 5 (Z)-1-(2-phenylhydrazono) naphthalen-2(1H)-one acts as a bidentate ligand. The IR, NMR (1D & 2D), ESI+ Mass spectrum and electronic studies confirm that the ligand coordinated to metal through oxygen and nitrogen as donor atoms. This ligand is used to synthetize copper (II), cobalt (II) and bismuth (III) complexes. The results of this investigation support the suggested structures of the metal complexes in which the copper (II) complex exhibits a tetrahedral geometry, whereas cobalt (II) and bismuth (III) complexes show an octahedral coordination. In vitro antibacterial proprieties were evaluated against four bacterial strains (three Gram-negative, one Gram-positive). All compounds showed activity against bacteria; however, metal complexes have a higher activity than the non-complexed ligand.
Conflict of Interests
- The authors declare that they have no conflict of interests.
ACKNOWLEDGMENTS
- Emmanuel Sopbué Fondjo warmly thanks the financial support of the DAAD (grant n° 91691265). Additional financial support was obtained from the research grant committee of the University of Dschang and the Cameroonian Higher Education Ministry.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML