-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2023; 13(2): 33-35
doi:10.5923/j.chemistry.20231302.01
Received: Feb. 17, 2023; Accepted: Feb. 27, 2023; Published: Mar. 2, 2023

On the Mechanism of the Hoppe-Seyler Test for Xanthine
Francisco Sánchez-Viesca, Reina Gómez
Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico
Correspondence to: Francisco Sánchez-Viesca, Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Xanthine, a dioxopurine, is a natural component of human urine. It was first isolated from a urinary calculus. This important biochemical substance can be detected by the colour test proposed by Hoppe-Seyler. He employed calcium chlorohypochlorite in alkaline medium, a green colour is observed with xanthine. Since the reaction pathway has not been advanced, the reaction mechanism of each step is provided. A redox reaction takes place after halogenation and alkaline hydrolysis via a variant of the Hofmann reaction. A nitrene is produced and reacts with the double bond in the uracil ring. A dipolar intermediate reacts with water, and the resulting imidzoline is hydrated by alkaline hydrolysis of the imino group in this cyclic amidine. A carbinolamide and a carbinolamine are formed whose isomerization gives rise to oxo derivatives and chain formation by ring opening. A second chlorination takes place, followed by dehydrohalogenation and isomerization. Assisted decarboxylation yields the final product, 5-ureidohydantoin.
Keywords: Calcium chlorohypochlorite, Carbinolamine, Hofmann reaction, Imine alkaline hydrolysis, Nitrene, Redox reaction
Cite this paper: Francisco Sánchez-Viesca, Reina Gómez, On the Mechanism of the Hoppe-Seyler Test for Xanthine, American Journal of Chemistry, Vol. 13 No. 2, 2023, pp. 33-35. doi: 10.5923/j.chemistry.20231302.01.
1. Introduction
- Xanthine is a two-oxygen derivative of adenine and guanine, the purine bases of DNA. Uric acid is a three-oxygen derivative of purine, and caffeine and theobromine are methyl xanthines.Xanthine, 2,6-dihydroxypurine, was first isolated from a urinary stone. It has since isolated from muscle, liver, and tea leaves, and it is a natural component of human urine, [1].A xanthine isomer is alloxanthine, a pyrazolopyrimidine, Figure 1.
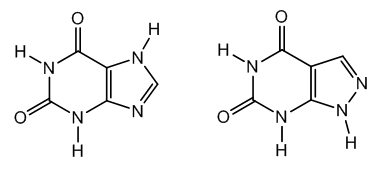 | Figure 1. Xanthine and alloxanthine structures |
2. Antecedents
- Xanthine is a purine base. Like uric acid, it gives the murexide reaction [7], but by different route.Traube prepared xanthine from 4,5-diamino-uracil by condensation with formic acid, [8,9].Uracil is 2,6-dioxo-tetrahydropyrimidine, and it is a constituent of the vegetal nucleic acids, [10].Felix Hoppe-Seyler (1825-1895), German physiologist and chemist, detected xanthine by reaction with calcium chlorohypochlorite in alkaline medium, giving green colour, [11-13]. He was the principal founder of biochemistry and molecular biology.The test is as follows: add the substance to be tested to a mixture of chlorinated lime and sodium hydroxide in a porcelain dish. A dark green ring is formed at first, quickly changing to brown, and finally disappearing.
3. Discussion
- Xanthine has two fused rings, a dioxopyrimidine (uracil), and an imidazole, also known as glyoxaline due to its preparation from glyoxal, ammonia, and formaldehyde, [14].The acidic hydrogen of the imido group in xanthine reacts with hypochlorite anion giving hypochlorous acid. This acid generated in situ is the reactive species for N-chlorination, [15,16].The hydrogen at N-3 is rather unavailable since a lactime (imidol) structure contributes to an α,β,γ,δ-unsaturated carbonyl, Figure 2.
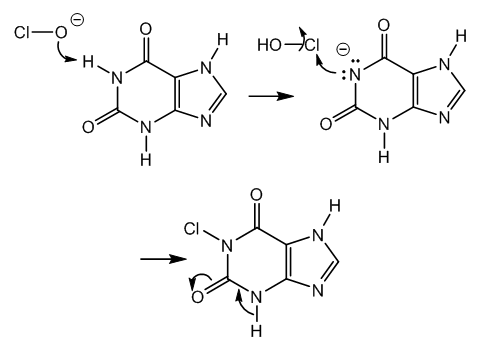 | Figure 2. Xanthine halogenation and isomerization |
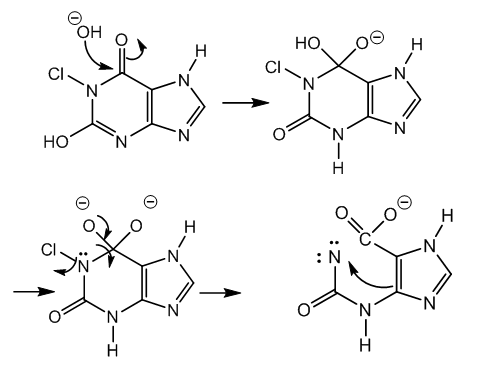 | Figure 3. Chlorimide hydrolysis and nitrene formation |
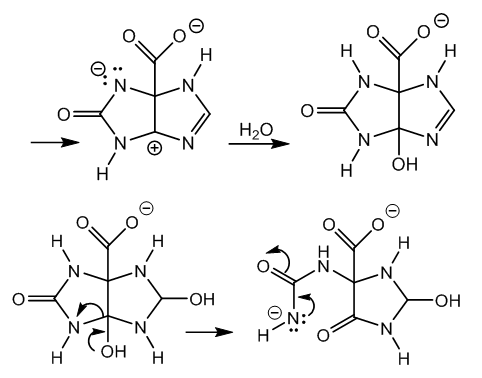 | Figure 4. Cleavage of carbinolamide and anion stabilization |
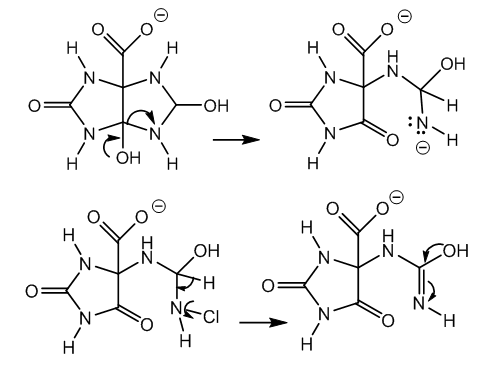 | Figure 5. Ring fission from carbinolamine, chlorination, and dehydrohalogenation |
 | Figure 6. 5-Ureidohydantoin by carbon dioxide release from β-carbonyl carboxylate |
4. Conclusions
- The mechanism of xanthine oxidation by means of calcium chlorohypochlorite in alkaline medium has been provided. This oxidation occurs via halogenation of the imido group, followed by hydrolysis of the haloimide. A nitrene results by ring opening and halide elimination, a variant of the Hofmann reaction, with concomitant formation of carboxylate. The nitrene attracts an electron pair from the double bond. The dipolar intermediate is neutralized by water, and an imidazoline ring results. The imine of this cyclic amidine is hydrated by alkaline hydrolysis. A carbinolamide and a carbinolamine are formed. The first one gives a carbonyl and an ureido chain. The latter produces a carbonyl and an oxidable chain. In both cases the final product is 5-ureidohydantoin via assisted removal of the carboxylate.
ACKNOWLEDGEMENTS
- Thanks are given to Martha Berros for support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML