-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2023; 13(1): 11-25
doi:10.5923/j.chemistry.20231301.02
Received: Dec. 12, 2022; Accepted: Jan. 5, 2023; Published: Jan. 13, 2023

Quantitative Nuclear Magnetic Resonance Spectroscopy in Pharmaceutical Chemistry: A Boon for Real Time Drug Detection!
Sudhaunshu S. Purohit
ETS Laboratories, USA
Correspondence to: Sudhaunshu S. Purohit, ETS Laboratories, USA.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Throughout the history of mankind numerous kinds of diseases played a contributing part in shaping the humanity. Since, the beginning of 21st century, the persistent efforts in the medical sciences by the teams of research scientists have witnessed the fruition in the form of vaccinations as well as various lifesaving pharmaceutical drugs with confirmed capability against several ailments. The development of a remedial drug, is equally important to that of its delivery system in human body at the intended location and subsequently nano-particles emerged as an excellent option surpassing the conventional ways. It is equally crucial to study the pharmacokinetics in order to establish the release profile of a drug from its nano-particle encasing along with the encapsulation capacity of the later. A few spectroscopic techniques have been proven to be effective for the quantitative analysis study and the best of them being NMR spectroscopy. This scientific review article successively describes an interdepartmental collaborative translational research including the extensive use of both solid state and solution state Nuclear Magnetic Resonance spectroscopy for the quantitative determination (qNMR) of an anti-HIV, phosphorous containing pharmaceutical drug (Tenofovir) encased in spray dried, mucoadhesive, pH sensitive, nano-particle casing composed of bio-degradable material such as alginate & thiolated chitosan along with the invitro pharmacokinetic study of its subsequent release profile when in contact with simulated human body fluids.
Keywords: Bacterial & Viral Diseases, Quantitative Solid State NMR Spectroscopy, Phosphorous, Drug Delivery & Detection Systems, Real Time Analysis, in-vitro Pharmacokinetics of Release Profile, Encapsulation Efficiency, Mathematical Models, Spray Dried, Mucoadhesive, pH Sensitive, Mannose Responsive, Alginate, Thiolated Chitosan, Hyaluronic acid, Nano-Formulations, HIV-AIDS, Tenofovir, International Conference of Harmonization, Method Development & Validation, Translational Research
Cite this paper: Sudhaunshu S. Purohit, Quantitative Nuclear Magnetic Resonance Spectroscopy in Pharmaceutical Chemistry: A Boon for Real Time Drug Detection!, American Journal of Chemistry, Vol. 13 No. 1, 2023, pp. 11-25. doi: 10.5923/j.chemistry.20231301.02.
1. Introduction
- It is a well-accepted fact that for millennia various types of bacterial and viral diseases played a devastating role in the evolution of human society. Several infectious and autoimmune diseases have afflicted human race since, the dawn of civilization in the known history. In 14th century epidemics such as plague commonly known as Black Death factually demonstrated the potential to cause mass extinction of human population in Europe. In the past two decades of 21st century the global human population witnessed a myriad of major viral disease’s pandemics caused by multitude of viruses such as flavi, alpha, filo, myxo, noro and corona to name a few. The recent COVID-19 situation which was imposed impromptu; factually demonstrated its influence to not only wipe out the lives of millions but also severely cripple the world economy.A significant development in the medical sciences promised greater life expectancy as either a cure or acute preventive therapies were invented to triumph over large number of medical conditions thus marking an introduction of a new era in the beginning of 20th century. The commendable amount of development in medical sciences since the early 20th century introduced a large number of lifesaving drugs which succeeded in putting a leash on the dispersal of majority of life threatening diseases. In spite of these accomplishments, to the date, a therapy is still far from curing the notorious diseases such as Alzheimer, Cancer, Hepatitis, AIDS etc. A successful vaccine is yet to be developed, which renders, an execution of precautionary measures to inhibit from infection to be the best strategy of survival. Substantial levels of physical and emotional alteration and a possible distortion to the ailing person’s personality as well as perspective on life are the direct repercussions ascribed to the contraction of these dire diseases.In the beginning of current century the scientific community worldwide remarkably got engaged in an intense research to invent measures, if not cure, to prevent the infection from these diseases and their further spread in human body after being diagnosed. Since then, an investigation to discover and develop an effectively functional drug against these diseases along with an efficient drug delivery system to administer the drug in infected human body is incessantly underway. The perseverance demonstrated by the scientific teams around the globe have now resulted in formulating certain drugs with proven ability against these diseases. Interestingly, majority of these drugs in pharmaceutical-medicinal field contain some of the most frequently characterized heteroatoms such as Phosphorous, Fluorine, Sulfur, & Boron along with commonly observed Nitrogen & Oxygen.As a matter of fact, it is not only the drugs but also their delivery systems in human body that are exclusively being researched and are under development. The extensive research in the field of nanotechnology has provided scientific community with a plausible solution to overcome the problems faced while developing suitable drug delivery system. Years of tenacious research has finally yielded in the development of a means to resolve problems associated with delivery of therapeutic compound to the target site. Nano-formulations are now being synthesized using numerous types of materials which are designed to release the encased drug strictly when triggered by change in the pH of the surrounding media. Hence, it is also crucial to understand the encapsulation capacity of these nano-formulations in their solid form along with the pharmacokinetics of their release profile from their nano-particle placebo-casing.A. The direst diseases of current era mentioned above and the pharmaceutical drugs contributing in their preventive treatment are discussed herein.A.1. Alzeimer’s Disease - Alpha GPCA.1.a. Severity of Alzeimer’s DiseaseAlzeimer’s disease was first identified, studied and reported by a German psychiatrist, Alois Alzheimer during years 1901-1906 [1-3]. It is a chronic neurodegenerative disease and is a major cause of dementia. In early stages, it indicates symptoms like short term memory loss which slowly but eventually culminates in permanent dementia. Other symptoms such as problems with language, disorientation, mood swings, loss of motivation, lack of self-care and many behavioral issues are observed in affected people during the advancement of the disease in body. People suffering from Alzeimer’s disease are often seen withdrawing from family and society as their condition exacerbates. Ultimately, by losing their bodily functions, they succumb to death [4-6]. In 2010, about 21-35 million cases of Alzheimer’s disease were identified worldwide, along with, about 486000 deaths that were reported as a result of dementia whereas, in 2020, approximately 50 million people worldwide were identified with Alzheimer's disease [5,7,8]. Even though, the exact cause of Alzheimer’s disease is unknown, 5% of the cases have been identified as genetics, whereas, several competing hypothesis such as cholinergic, amyloid, tau hypothesis etc. exist, trying to explain the causes for the remaining cases [9].A.1.b. Role of Alpha GPC and its Administration
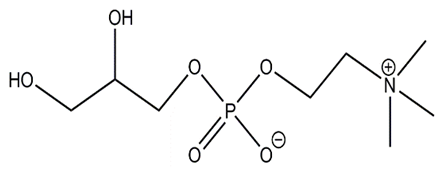 | Figure 1. Alpha-GPC |
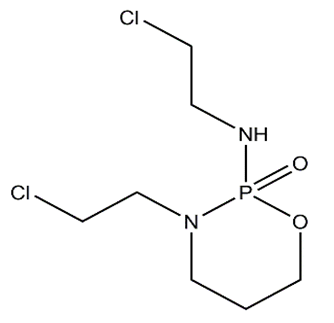 | Figure 2. Ifosfamide |
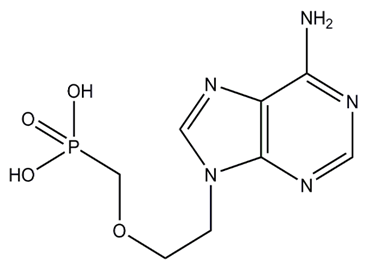 | Figure 3. Adefovir |
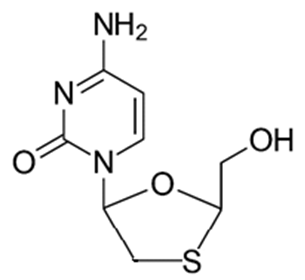 | Figure 4. Lamivudine |
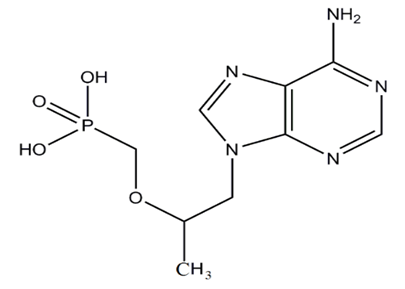 | Figure 5. Tenofovir |
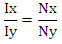 The molar ratio Nx/Ny of two compounds X and Y can be calculated straightforward using-
The molar ratio Nx/Ny of two compounds X and Y can be calculated straightforward using-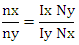 Consequently, the amount fraction of a compound X in a mixture of m components is given by-
Consequently, the amount fraction of a compound X in a mixture of m components is given by-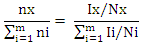 without any need to consider the solvent signal in which the mixture is dissolved as the only sample preparation step. For the purity determination of a substance an internal standard with known purity is needed. The purity of the analyte Px can be calculated as follows-
without any need to consider the solvent signal in which the mixture is dissolved as the only sample preparation step. For the purity determination of a substance an internal standard with known purity is needed. The purity of the analyte Px can be calculated as follows-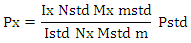 Here, Mx and MStd are the molar masses of the analyte and the standard, respectively, ‘m’ the weighed mass of the investigated sample, mStd and PStd are the weighed mass and the purity of the standard and NStd and IStd correspond to the number of spins and the integrated signal area of a typical NMR line of the standard, as described above.Taking into account the theory & principle of quantitative NMR an advancement in the research project involving the study of a pharmaceutical drug encased in a nano-formulation can be based on certain definite aims-Aim 1: Development and validation of a method focused on a specific NMR active nuclei constituting a pharmaceutical drug molecule of interest for the quantitative analysis study utilizing both solid state and solution state NMR spectroscopic techniques.Aim 2: Application of solution state NMR spectroscopic technique to establish pharmacokinetics of in vitro drug release from the nano-formulation in various simulated human body fluids.Aim 3: Application of solid state NMR spectroscopic technique to determine the encapsulation efficiency of a drug and nano-formulation pair.Aim 4: Continue and expand with an investigation of a variety of pharmaceutical drug molecules with proven ability in the treatment of various calamitous diseases of current century such as Alzheimer, Cancer, Hepatitis, AIDS etc. utilizing proposed qNMR spectroscopic methods. E.3. Advances in Detection Technique Research has been carried out where quantitative nuclear magnetic resonance (qNMR) spectroscopy was employed to analyze the drug under study by taking advantage of the NMR active nuclei with larger % natural abundance such as Hydrogen, Phosphorous or Fluorine atom in the drug molecule. 1H solution state NMR method to perform a real-time detection and quantification of Tenofovir have been developed and validated [119]. The drug release profile was established in simulated vaginal fluid solution and vaginal-seminal fluid solution by integrating the two peaks (δ = 8.00 & 8.09 ppm) generated from the aromatic protons in Tenofovir to quantify its respective amount. Their results have demonstrated a good sensitivity as well as specificity that allowed the direct quantification of in vitro Tenofovir release from a spray-dried mucoadhesive and pH-sensitive MS formulation based on polymethacrylate salt without the membrane diffusion technique. Advancement in the research was focused primarily on the development and implementation of a general qNMR method specifically focused on 31P which is NMR active nuclei to achieve direct, real time quantification of in vitro drug release. Researcher stood up in an effort aimed to provide medical field with a better understanding of the drug loading and drug releasing profiles of the large number of phosphorus containing drugs [120-123]. Both solution-state & solid-state NMR spectroscopic techniques were utilized to establish the pharmacokinetics of drug release and for the determination of encapsulation efficiency of nano-formulation for a particular drug. Tenofovir, an antiretroviral topical microbicide with proven mettle against HIV/AIDS was chosen as model phosphorous containing drug. The proven effectiveness of a specific type of spray dried, mucoadhesive, pH sensitive, mannose responsive nano-formulations composed of bio-degradable material, alginate & thiolated chitosan, intended to serve as an encasing for Tenofovir was selected.E.3.a. Synthesis of Nano-Particle Casings- The NPs were synthesized by alternatively coating alginate and thiolated chitosan over Tenofovir core by a layer by-layer method. Sprayed alginate NPs to be suspended into the solution of CaCl2 and TCS and stirred. The product was collected by centrifugation and washed thoroughly with water. NPs were then suspended in aqueous solution of alginate and the respective product to be collected by centrifugation and washed thoroughly with water. This process was repeated ‘n’ times in order to obtain, single, double and triple layered NPs (SLNP, DLNP, TLNP) as the final product.The research is primarily focused on the development and validation of a 31P-qNMR method that can be implemented to determine the amount of drug released from its placebo-encasing in the simulated human body fluids such as simulated plasma, vaginal & seminal fluids. Considering the drawbacks of 1H-NMR & 13C-NMR assays, 31P-qNMR is an excellent technique for studying phosphorus containing compounds. Phosphorous with nuclear spin ½, comparatively higher gyromagnetic ratio and natural isotopic abundance of 100%, produces high signal-to-noise (S/N) ratio, thus making 31P-qNMR method considerably economical and 379 times more sensitive.
Here, Mx and MStd are the molar masses of the analyte and the standard, respectively, ‘m’ the weighed mass of the investigated sample, mStd and PStd are the weighed mass and the purity of the standard and NStd and IStd correspond to the number of spins and the integrated signal area of a typical NMR line of the standard, as described above.Taking into account the theory & principle of quantitative NMR an advancement in the research project involving the study of a pharmaceutical drug encased in a nano-formulation can be based on certain definite aims-Aim 1: Development and validation of a method focused on a specific NMR active nuclei constituting a pharmaceutical drug molecule of interest for the quantitative analysis study utilizing both solid state and solution state NMR spectroscopic techniques.Aim 2: Application of solution state NMR spectroscopic technique to establish pharmacokinetics of in vitro drug release from the nano-formulation in various simulated human body fluids.Aim 3: Application of solid state NMR spectroscopic technique to determine the encapsulation efficiency of a drug and nano-formulation pair.Aim 4: Continue and expand with an investigation of a variety of pharmaceutical drug molecules with proven ability in the treatment of various calamitous diseases of current century such as Alzheimer, Cancer, Hepatitis, AIDS etc. utilizing proposed qNMR spectroscopic methods. E.3. Advances in Detection Technique Research has been carried out where quantitative nuclear magnetic resonance (qNMR) spectroscopy was employed to analyze the drug under study by taking advantage of the NMR active nuclei with larger % natural abundance such as Hydrogen, Phosphorous or Fluorine atom in the drug molecule. 1H solution state NMR method to perform a real-time detection and quantification of Tenofovir have been developed and validated [119]. The drug release profile was established in simulated vaginal fluid solution and vaginal-seminal fluid solution by integrating the two peaks (δ = 8.00 & 8.09 ppm) generated from the aromatic protons in Tenofovir to quantify its respective amount. Their results have demonstrated a good sensitivity as well as specificity that allowed the direct quantification of in vitro Tenofovir release from a spray-dried mucoadhesive and pH-sensitive MS formulation based on polymethacrylate salt without the membrane diffusion technique. Advancement in the research was focused primarily on the development and implementation of a general qNMR method specifically focused on 31P which is NMR active nuclei to achieve direct, real time quantification of in vitro drug release. Researcher stood up in an effort aimed to provide medical field with a better understanding of the drug loading and drug releasing profiles of the large number of phosphorus containing drugs [120-123]. Both solution-state & solid-state NMR spectroscopic techniques were utilized to establish the pharmacokinetics of drug release and for the determination of encapsulation efficiency of nano-formulation for a particular drug. Tenofovir, an antiretroviral topical microbicide with proven mettle against HIV/AIDS was chosen as model phosphorous containing drug. The proven effectiveness of a specific type of spray dried, mucoadhesive, pH sensitive, mannose responsive nano-formulations composed of bio-degradable material, alginate & thiolated chitosan, intended to serve as an encasing for Tenofovir was selected.E.3.a. Synthesis of Nano-Particle Casings- The NPs were synthesized by alternatively coating alginate and thiolated chitosan over Tenofovir core by a layer by-layer method. Sprayed alginate NPs to be suspended into the solution of CaCl2 and TCS and stirred. The product was collected by centrifugation and washed thoroughly with water. NPs were then suspended in aqueous solution of alginate and the respective product to be collected by centrifugation and washed thoroughly with water. This process was repeated ‘n’ times in order to obtain, single, double and triple layered NPs (SLNP, DLNP, TLNP) as the final product.The research is primarily focused on the development and validation of a 31P-qNMR method that can be implemented to determine the amount of drug released from its placebo-encasing in the simulated human body fluids such as simulated plasma, vaginal & seminal fluids. Considering the drawbacks of 1H-NMR & 13C-NMR assays, 31P-qNMR is an excellent technique for studying phosphorus containing compounds. Phosphorous with nuclear spin ½, comparatively higher gyromagnetic ratio and natural isotopic abundance of 100%, produces high signal-to-noise (S/N) ratio, thus making 31P-qNMR method considerably economical and 379 times more sensitive. 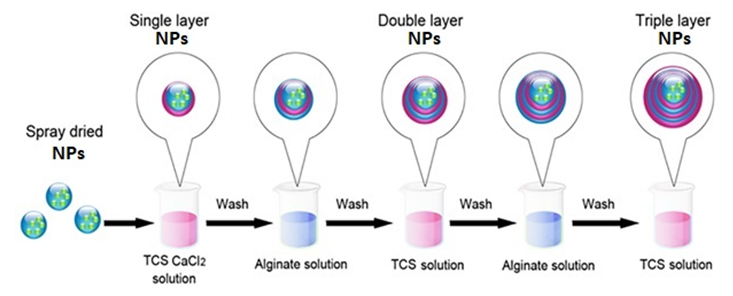 | Figure 6. Schematic representation of preparation of multilayer nano-particles (NPs) of thiolated chitosan and alginate alternately layered over a core containing Tenofovir by electro-spinning method |
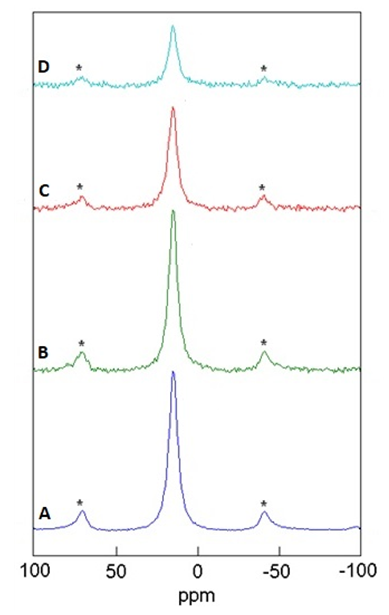
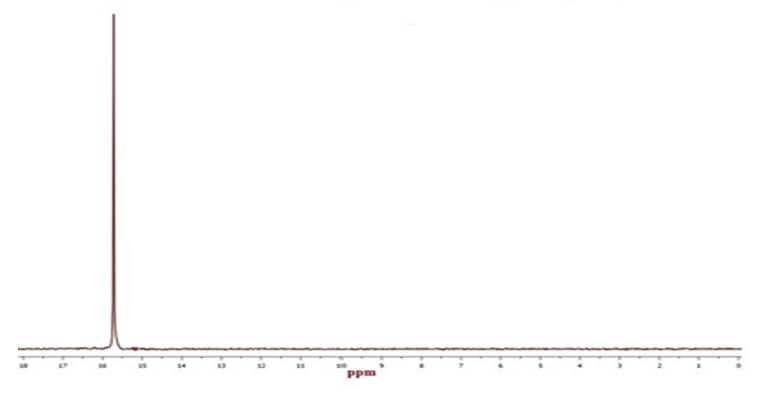 | Figure 7. 31P solution state NMR spectra of pure Tenofovir (δ = 15.7 ppm) in simulated vaginal fluid |
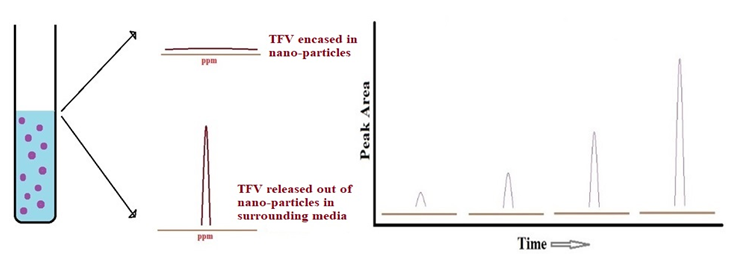 | Figure 8. Schematic representation of the release of Tenofovir from its nano-particle casing in simulated human body fluids by 31P solution state qNMR spectroscopy |
2. Conclusions
- Solution state NMR spectroscopic technique can be implemented to establish real time kinetics of in vitro drug release from its nano-formulation casing in simulated human body fluids such as seminal & vaginal fluids and blood plasma. On the other hand, solid state NMR spectroscopic technique can be employed to determine the encapsulation efficiency of a nano-formulation for the drug under study. In general, these particular research projects are very motivating as they contribute towards that one step which brings scientific community closer towards inventing effective treatment on HBV-Hepatitis B, HIV-AIDS and other similar dire diseases. Hence, working on such projects for any scientist proves to be the principle key in achieving the greatest goal of their career that they incessantly pursued throughout many years which is a progressive role in the future of humanity through scientific inventions. In 21st century, the scientific institutions in many developing nations are positively impacting the health of millions around the globe as they contribute by investing in the research & development of such innovative technologies. Human species have been deeply affected by multiple life threatening diseases for ages; a need of time it is now to rattle their cage.
ACKNOWLEDGEMENTS
- The NMR experiments of an interdepartmental collaborative translational research presented herein were conducted at University of Missouri Kansas City (UMKC). The author of this manuscript cordially appreciates the research work contributed by Dr. Vivek Agrahari, Dr. Jianing Meng, Dr. Fohona Coulibaly and Prof. Bi-Botti Youan of School of Pharmacy, UMKC and is also immensely grateful to Prof. Nathan Oyler of Department of Chemistry, UMKC for his invaluable guidance.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML