-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2023; 13(1): 1-10
doi:10.5923/j.chemistry.20231301.01
Received: Dec. 11, 2022; Accepted: Dec. 26, 2022; Published: Jan. 13, 2023

Larvicidal and Ovicidal Activities of Some Cinnamaldehyde Derivatives against Anopheles Gambiae, Malaria Vector Agent
Victor N’goka1, 2, Guy Crépin Enoua1, Ghislain Kende1, 2, Tony Wheellyam Pouambeka1, Ninon G. E. R. Etsatsala3
1Unit of Plant and Life Chemistry, Faculty of Science and Technology, Marien Ngouabi University, Brazzaville, Republic of the Congo
2Laboratory of Medicinal Chemistry and Pharmacotechnie of Medicinal Plants, CHIRED-CONGO 81, Dahomey Street Poto-Poto, Brazzaville, Republic of the Congo
3Department of Horticultural Sciences, Applied Sciences Faculty, Cape Peninsula University of Technology, Symphony Road, Bellville, Cape Town, South Africa
Correspondence to: Victor N’goka, Unit of Plant and Life Chemistry, Faculty of Science and Technology, Marien Ngouabi University, Brazzaville, Republic of the Congo.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Main research studies have been conducted on vector control for the fight against malaria, a very devastating disease in sub-Saharan Africa. This study was designed to carry out larvicidal and ovicidal activities of some chemical constituents of essential oils. Eleven compounds including related derivatives have been either purchased or synthesized and evaluated for their activities. Larvae and eggs of anopheles have been produced. Cinnamaldehyde and ortho-Nitrocinnamaldehyde exhibited the highest activity with LC50 = 55 mg /L against larvaes. Furthermore, Citral, and Cinnamaldhyde showed the highest activity against eggs, with LC50 = 0.015 and 0.020 respectively. This study revealed that the compounds were more active than their corresponding essential oils. Hence, it is important to carry out a more in-depth study of the structure activity relationship on chemical compounds was been demonstrated.
Keywords: Cinnamaldehyde derivatives, Essential oil constituents, Larvicidal, Ovicidal activities
Cite this paper: Victor N’goka, Guy Crépin Enoua, Ghislain Kende, Tony Wheellyam Pouambeka, Ninon G. E. R. Etsatsala, Larvicidal and Ovicidal Activities of Some Cinnamaldehyde Derivatives against Anopheles Gambiae, Malaria Vector Agent, American Journal of Chemistry, Vol. 13 No. 1, 2023, pp. 1-10. doi: 10.5923/j.chemistry.20231301.01.
1. Introduction
- Malaria remains one of the most devastating endemics in black Africa since time immemorial. It is caused by a Hemococcidae of the genus Plasmodium, transmitted to humans by a female mosquito of the genus Anopheles. The world Health Organization (WHO) statistically reported that an approximation of 627000 deaths related to malaria occurred in 2020, of which 96% were recorded in Africa. Additionally, children under 5 years and pregnant women were the most vulnerable segment of the population [1].Several efforts have been made to combat this disease, including the use of vector control and other products such as DDT, which ended up being ban because of their detrimental effects on the environment [2,3,4,5]. However, resistance to antimalarials drugs has been observed for the past decade and we are gradually returning to vector control, with low environmental effects through intensive research on biopesticides [6,7,8]. Thus, several works investigated the activities of essential oils against Anopheles gambiae [9,10,11,12,13,14,15]. Mdoe et al. 2014, reported that the essential oils of Cinnamomum osmophloeum exhibited high mortality of A. gambiae s.s. third instar larvae dosage and time dependant in both laboratory and semi-field trials [9]. Furthermore, several other authors including Bossou, et al. 2013, reported that the essential oils from Cymbopogon. Citrates have insecticidal properties against the vector of malaria, Anopheles gambiae [10].The use of pesticides derived from plant materials may create another problem with regard to the conservation of biodiversity. Indeed, it seems impossible to reconcile the high demand for raw materials of plant origin for industry and the respect of conventions and treaties on the conservation of biodiversity [16]. The history of malaria tells us that the first natural drug was quinine, which gave rise to chloroquine [17]. Antibiotic therapy also began with molecules from biosynthesis to arrive at synthetic antibiotics [17]. Literature have showed that monoterpenes have activities against insects such as Aedes aegypti, Culex pipiens and Anopheles gambiae [18,19,20,21]. Newman et al reported on a 20, 25 and 30 years statistical studies comparing natural, hemiisynthetic and synthetic products. The founding of these studies showed that industrial products are predominantly synthesis molecules [22,23,24]. The history of the evolution of antimalarial and antibiotic drugs has shown that the natural substance led to the synthetic product (quinine and chloroquine for example). In addition, studies by Newman et al. showed that a lot of drugs in the market have synthetic origin. Several studies have shown that synthetic monoterpenes are more active than the essential oils from which they come. This research focused on the choice of synthetic molecules as chemical constituents of essential oils, and secondly on the evaluation of their larvicidal and ovicidal activities. The outcomes of this research study are directed to the finding of more bioactive compounds with significant quantity.
2. Experimental Section
- Materials and methodsAll chemicals and solvents were purchased from VWR-France. The melting points of synthesized compounds were determined in Kofler banc and are uncorrected. Thin layer chromatography (TLC) plate (silica gel G) were purchased from Merck and various solvents such as toluene, acetone, ethanol were used or combined to obtained the more effective and prominent solvent systems. Finally, the UV lamp were used to visualize the TLC plate at two different wavelengths: 254 and or 365 nm. The NMR spectra were carried out on an Avance 400 MHz spectrometer (Bruker, Rheinstetten, Germany), using deuterated dimethylsulfoxide.Study areaThis research project was conducted in 2020 in CHIRED – CONGO at the laboratory of medicinal chemistry and pharmacotechnie of medicinal plants, B.P: 13.922 Brazzaville, Republic of Congo and at the Plant and Life Chemistry Unit, Faculty of Sciences and Techniques, Marien Ngouabi University, Brazzaville BP 69.Chemical structures of selected compoundsTable 1 describes the molecular structures of Citral, Cinnamaldehyde, Thymol, Ionone, Menthone, Citronellal, ortho-Nitrocinnamaldehyde, Cinnamic acid, meta-Nitrocinnamic acid, Permethrin and Cypermethrin.
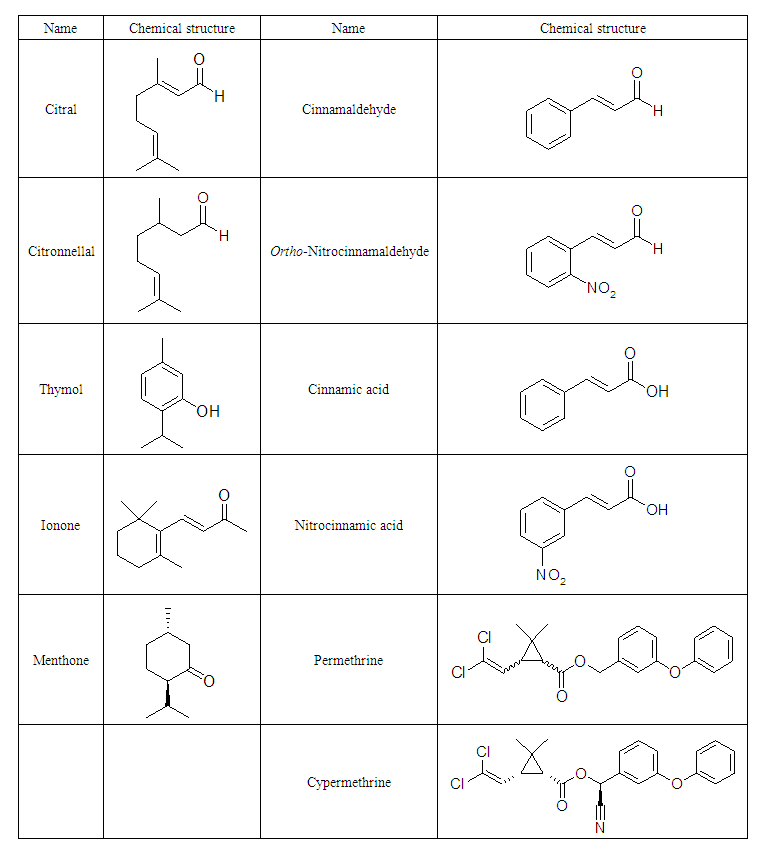 | Table 1. Names and chemical structures of compounds |
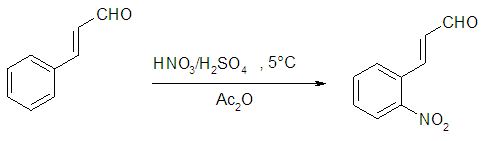 A mass of 2.6 g (20 mmol) of cinnamaldehyde was placed in a 500 mL three necked round bottom flask. A volume 25 mL of acetic anhydride was added to the flask. The balloon was put in an ice with salt bath, and then placed on a magnetic stirrer. A volume of 1 mL of nitric acid and 2.5 mL of sulphuric acid was added and stirred for further 18 min, then for 2 hours on the ice bath. The solution was allowed to cool down for 48 hours, and, the solution of concentrated hydrochloric acid was added to the reaction medium and allow to cool down to reveal the crystals. The precipitate obtained was filtered and dried. The product was recrystallized in ethanol to obtain pure ortho-Nitrocinnamaldehyde.Synthesis of cinnamic acid [26] and meta-Nitrocinnamic acid [27]
A mass of 2.6 g (20 mmol) of cinnamaldehyde was placed in a 500 mL three necked round bottom flask. A volume 25 mL of acetic anhydride was added to the flask. The balloon was put in an ice with salt bath, and then placed on a magnetic stirrer. A volume of 1 mL of nitric acid and 2.5 mL of sulphuric acid was added and stirred for further 18 min, then for 2 hours on the ice bath. The solution was allowed to cool down for 48 hours, and, the solution of concentrated hydrochloric acid was added to the reaction medium and allow to cool down to reveal the crystals. The precipitate obtained was filtered and dried. The product was recrystallized in ethanol to obtain pure ortho-Nitrocinnamaldehyde.Synthesis of cinnamic acid [26] and meta-Nitrocinnamic acid [27]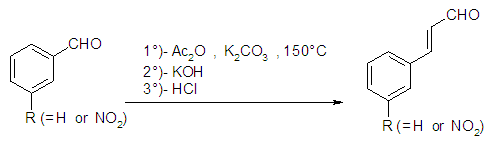 An amount of 3.75 g of anhydrous potassium carbonate and 12.5 mL (130 mmol) of acetic anhydride were placed in a properly equipped 250 mL three necked round bottom flask. The reaction medium was shacked, and 85 mmol of the corresponding aldehyde was added. The temperature of the mixture was increased to 150°C for 1 hour and 15 minutes while checking the foam. In a 500 mL beaker placed in an ice bath, prepare a solution of 14 g potassium hydroxide in 120 mL of water. After cooling slowly, the still warm to the reaction mixture (about 100°C) was pour into the beaker held in the ice bath. The solution was washed 2 times with 20 mL of diethyl ether. Acidify the aqueous phase with concentrated hydrochloric acid up to pH at about 1. Cool to a temperature below 10°C. The raw cinnamic acid was filtered on Buchner, then washed with cold water and recrystallized in water/ethanol mixture (2-1).Production of Anopheles gambiae’s eggsThe larvae of Anopheles gambiae were collected at the edge of the river Tsiémé, in Ouenzé - Brazzaville. These larvae were transported to the Faculty of Sciences and Technologies, where they were fed with non-creamy cookies for 4 days, in a cubic cage, 60 cm sides, surrounded by a non-impregnated mosquito net on their face, so that adult mosquitoes from the emergence of the larvae do not escape. Several kinds of cotton soaked in glucose solution were placed in the mosquito cage so that they could feed. After 3 days of feeding, the adult mosquitoes with glucose solution, an anaesthetized rat was introduced into the mosquito cage for 48 hours, so that the fertilized females take their blood meal for the good development of their eggs. After 4 days of the blood meal, mosquito eggs were laid in small plastic tubs, then placed in the mosquito box.Larvicidal and ovicidal activitiesLarvicidal and ovicidal bioassay was carried out as described by Kende et al. [14,15]. Larvicid and ovicide activities have reconciled counts of dead larvae or eggs after exposure to solutions of Citral, Cinnamaldehyde, Thymol, Ionone, Menthone and Citronellal, ortho-Nitrocinnamaldehyde, meta-Nitrocinnamic acid, Cinnamic acid, Permethrin and Cypermethrin. Several 25 larvae of Anopheles gambiae in stage 4 or 25 eggs were introduced into a 5 cm diameter cup containing 80 mL of tested product concentration solution. The concentrations were 0 ppm (control), 12.5, 25, 50, 100, 200 and 400 ppm except for Permethrin and Cypermethrin where concentrations were 0.05 g/L; 0.025 g/L; 0.0125 g/L; 0.0062 g/L and 0.0031 g/L prepared from 10% commercial solutions. The set is incubated at room temperature for 24 hours for larvae and 72 hours for eggs. All the experiments were repeated three times. The percentage of mortality of larvae was calculated using formula 1, while the percentage of mortality of eggs was calculated using formula 2, previously described in our previous works [14,15].
An amount of 3.75 g of anhydrous potassium carbonate and 12.5 mL (130 mmol) of acetic anhydride were placed in a properly equipped 250 mL three necked round bottom flask. The reaction medium was shacked, and 85 mmol of the corresponding aldehyde was added. The temperature of the mixture was increased to 150°C for 1 hour and 15 minutes while checking the foam. In a 500 mL beaker placed in an ice bath, prepare a solution of 14 g potassium hydroxide in 120 mL of water. After cooling slowly, the still warm to the reaction mixture (about 100°C) was pour into the beaker held in the ice bath. The solution was washed 2 times with 20 mL of diethyl ether. Acidify the aqueous phase with concentrated hydrochloric acid up to pH at about 1. Cool to a temperature below 10°C. The raw cinnamic acid was filtered on Buchner, then washed with cold water and recrystallized in water/ethanol mixture (2-1).Production of Anopheles gambiae’s eggsThe larvae of Anopheles gambiae were collected at the edge of the river Tsiémé, in Ouenzé - Brazzaville. These larvae were transported to the Faculty of Sciences and Technologies, where they were fed with non-creamy cookies for 4 days, in a cubic cage, 60 cm sides, surrounded by a non-impregnated mosquito net on their face, so that adult mosquitoes from the emergence of the larvae do not escape. Several kinds of cotton soaked in glucose solution were placed in the mosquito cage so that they could feed. After 3 days of feeding, the adult mosquitoes with glucose solution, an anaesthetized rat was introduced into the mosquito cage for 48 hours, so that the fertilized females take their blood meal for the good development of their eggs. After 4 days of the blood meal, mosquito eggs were laid in small plastic tubs, then placed in the mosquito box.Larvicidal and ovicidal activitiesLarvicidal and ovicidal bioassay was carried out as described by Kende et al. [14,15]. Larvicid and ovicide activities have reconciled counts of dead larvae or eggs after exposure to solutions of Citral, Cinnamaldehyde, Thymol, Ionone, Menthone and Citronellal, ortho-Nitrocinnamaldehyde, meta-Nitrocinnamic acid, Cinnamic acid, Permethrin and Cypermethrin. Several 25 larvae of Anopheles gambiae in stage 4 or 25 eggs were introduced into a 5 cm diameter cup containing 80 mL of tested product concentration solution. The concentrations were 0 ppm (control), 12.5, 25, 50, 100, 200 and 400 ppm except for Permethrin and Cypermethrin where concentrations were 0.05 g/L; 0.025 g/L; 0.0125 g/L; 0.0062 g/L and 0.0031 g/L prepared from 10% commercial solutions. The set is incubated at room temperature for 24 hours for larvae and 72 hours for eggs. All the experiments were repeated three times. The percentage of mortality of larvae was calculated using formula 1, while the percentage of mortality of eggs was calculated using formula 2, previously described in our previous works [14,15].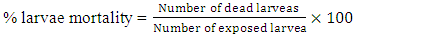 | (1) |
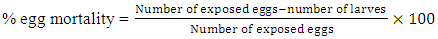 | (2) |
3. Resultats et Discussion
- Synthesis of ortho-NitrocinnamaldéhydeThe ortho-Nitrocinnamaldehyde was synthesized by nitration of Cinnamaldehyde under the action of nitric-sulphuric acid mixture in acetic anhydride at 5°C. The product was obtained with a yield of 50%, purity was confirmed by CCM and the melting point was 126°C, lit. 126-127.5°C [25]. C9H7O3N: 177,16 1H-MR (Fig. 1): 6.76 (dd, 1H, J = 15.8 Hz, J = 7.6 Hz, 1H, -CH=CH-CHO), 7.69 (td, 1H, J = 7.1 Hz, J = 1.1 Hz, H5-Ar); 7.77 (t, 1H, J = 7.2 Hz, 1H, H4-Ar), 7.90 (d, 1H, J = 7.3 Hz, H6 Ar), 7.99 (d, 1H, J = 15.8, -CH=CH-CHO), 8.06 (dd, 1H, J = 8.1 Hz, J = 0.9, H3 Ar), 9.69 (d, 1H, J = 7.6, -CHO); 13C-NMR (Fig. 2): 125.25 (C3 Ar), 129.44 (C1 Ar), 129.76 (Ar-CH=CH), 132.10 (C4 Ar), 132.44 (C5 Ar), 134.50 (C6 Ar), 147.88 (C2-NO2), 148.61 (Ar-CH=CH-), 195.10 (-CHO).
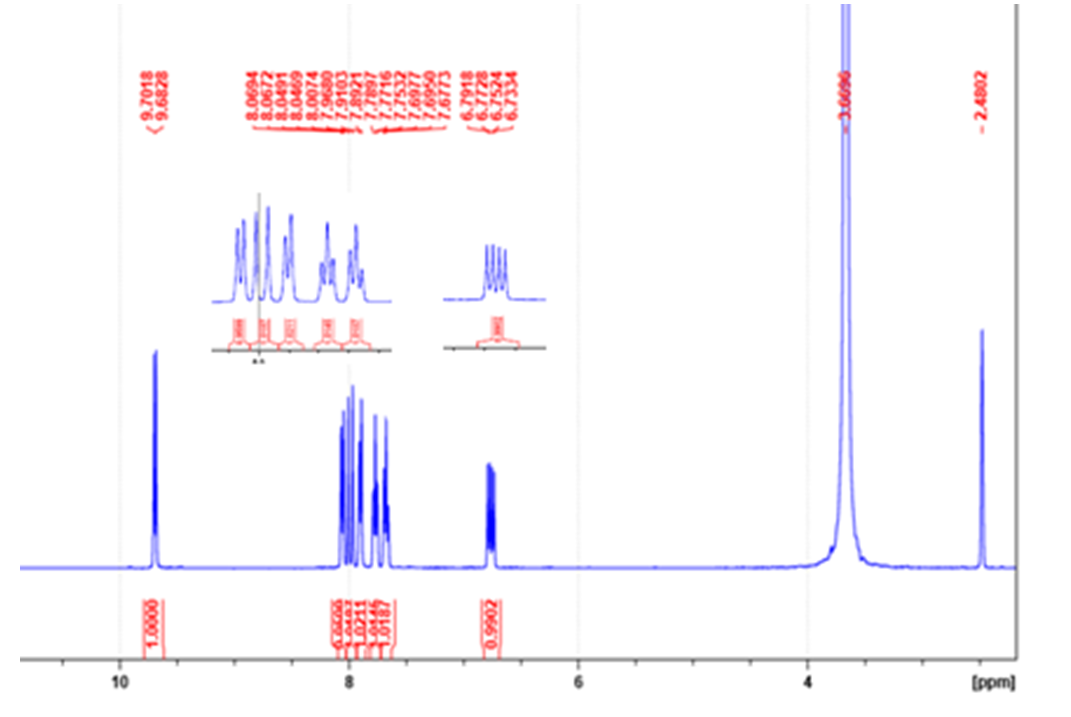 | Figure 1. 1H-NMR (400 MHz, DMSO-d6) of ortho-Nitrocinnamaldehyde |
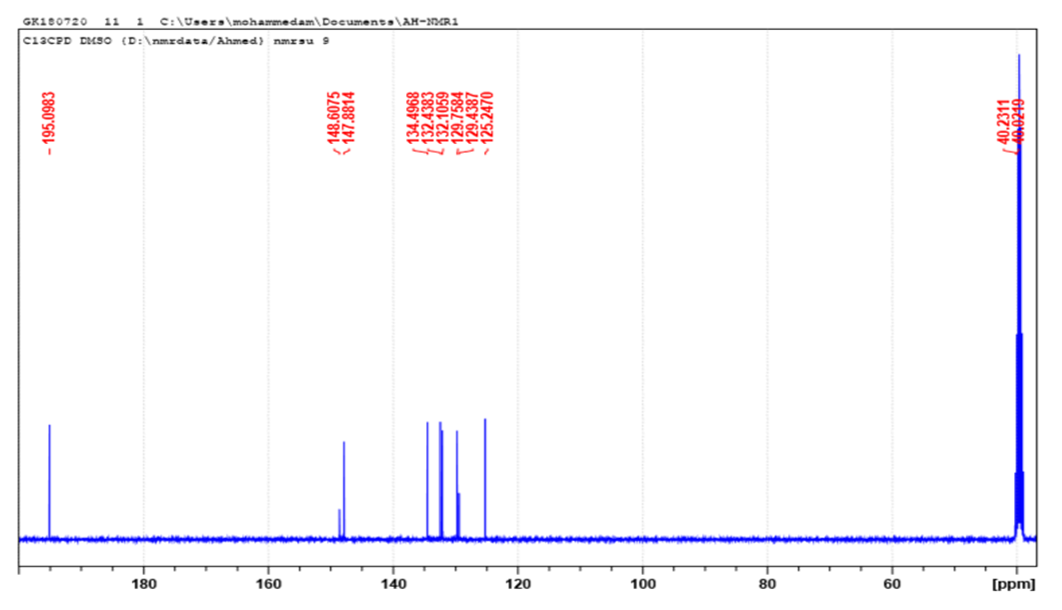 | Figure 2. 13C-NMR (DMSO-d6) of ortho-Nitrocinnamaldehyde |
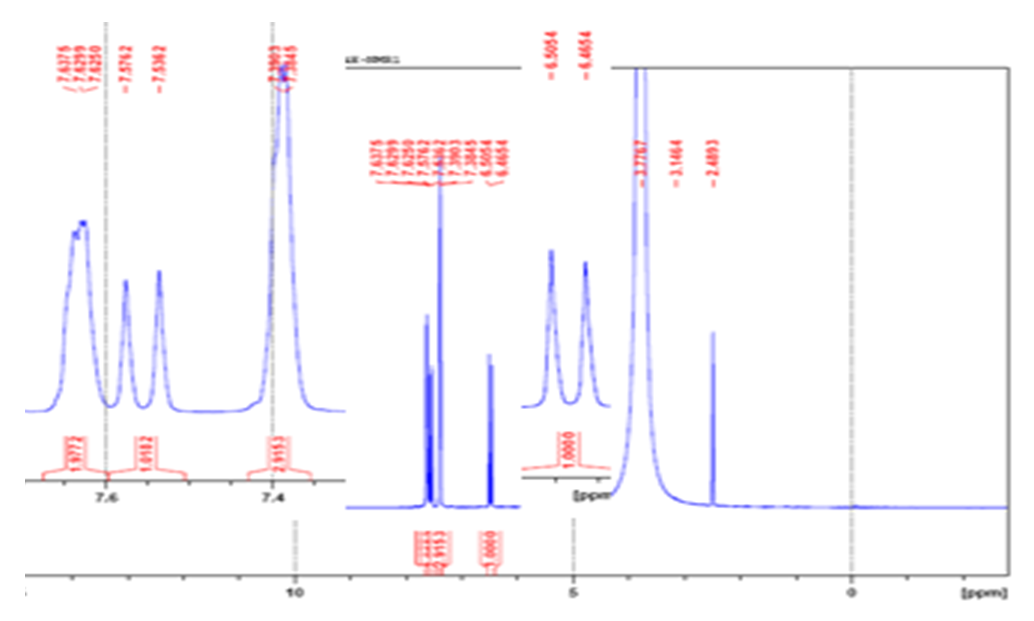 | Figure 3. 1H-NMR (400 MHz, DMSO-d6) of Cinnamic acid |
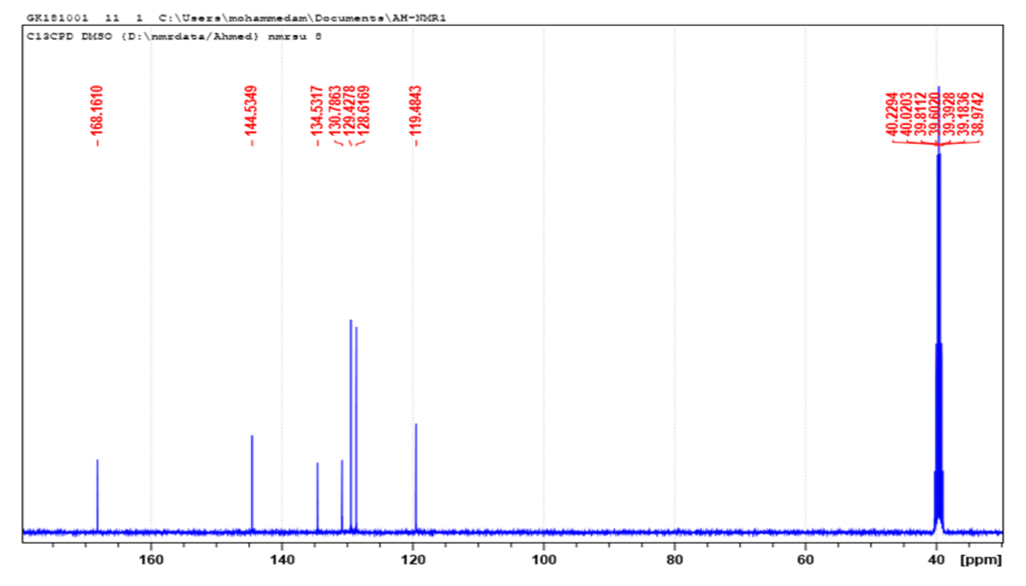 | Figure 4. 13C-MNR (DMSO-d6) of Cinnamic acid |
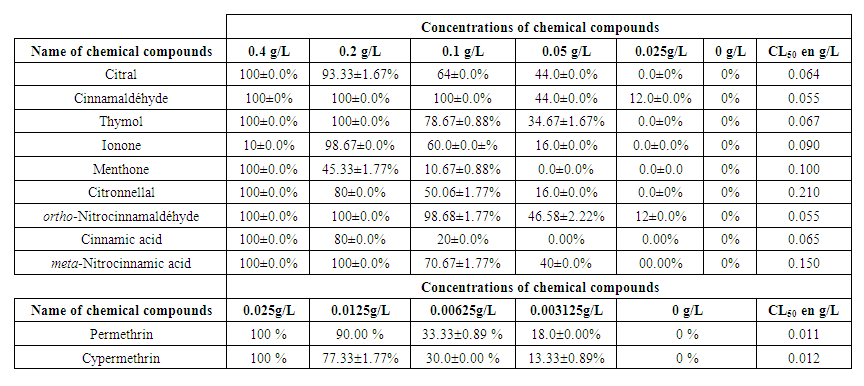 | Table 2. Mortality of larvae exposed to increasing concentrations of chemical compounds in percent and LC50 |
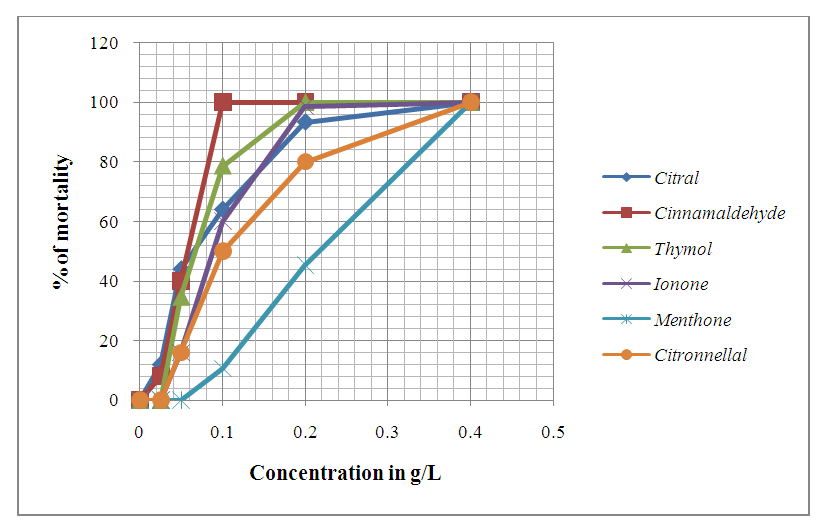 | Figure 5. Evolution of Anophèles gambiae larvae mortality after 24 hrs of exposition to different concentrations of chemical compounds |
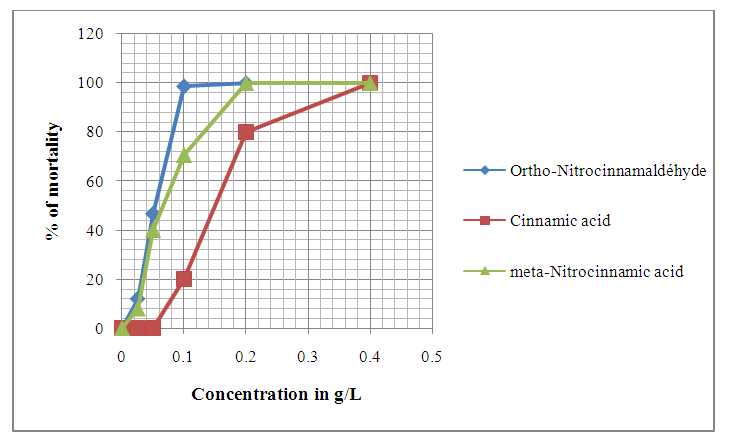 | Figure 6. Evolution of Anophèles gambiae larvae mortality after 24 hours of exposition to different concentrations of chemical compounds |
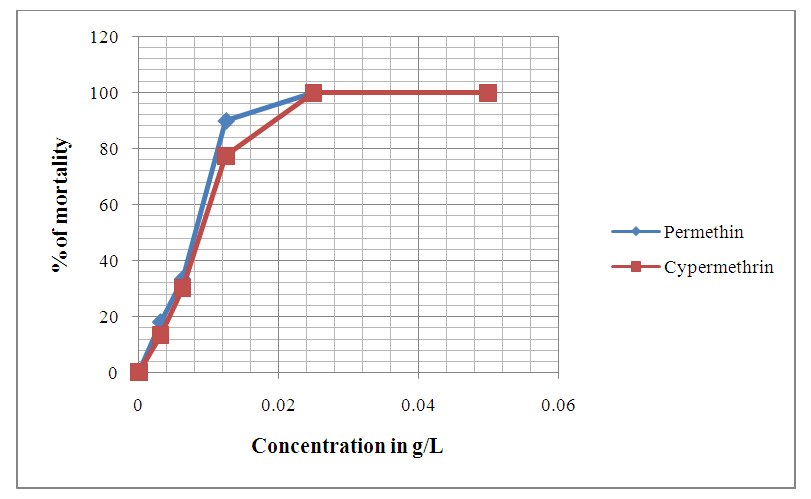 | Figure 7. Evolution of Anophèles gambiae larvae mortality after 24 hours of exposition to different concentrations of chemical compounds Permethrin and Cypermethrin |
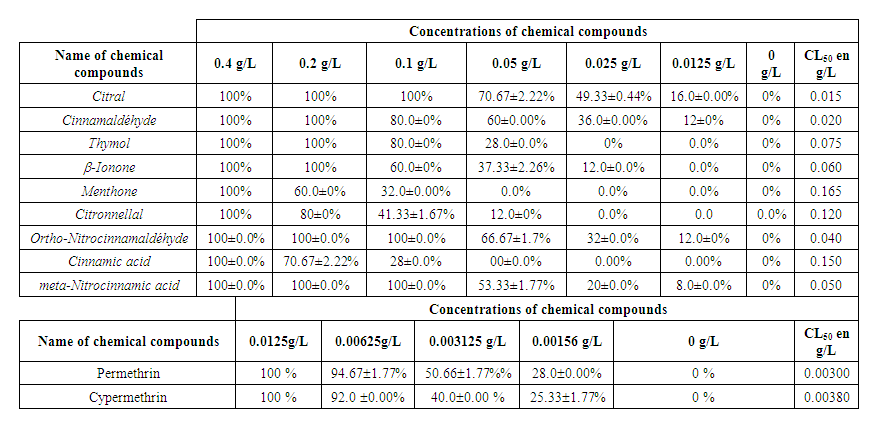 | Table 3. Mortality of eggs exposed to increasing concentrations of chemical compounds in percent and LC50 |
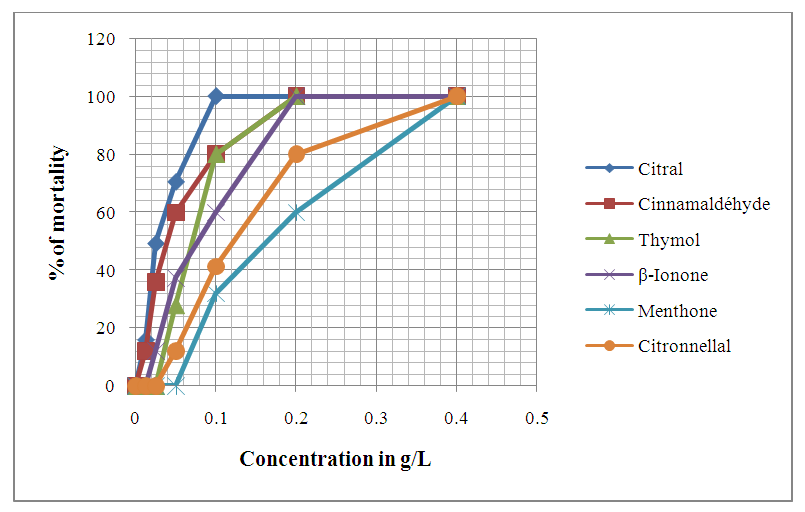 | Figure 8. Evolution of Anophèles gambiae eggs mortality after 72 hours of exposition to different concentrations of chemical compounds |
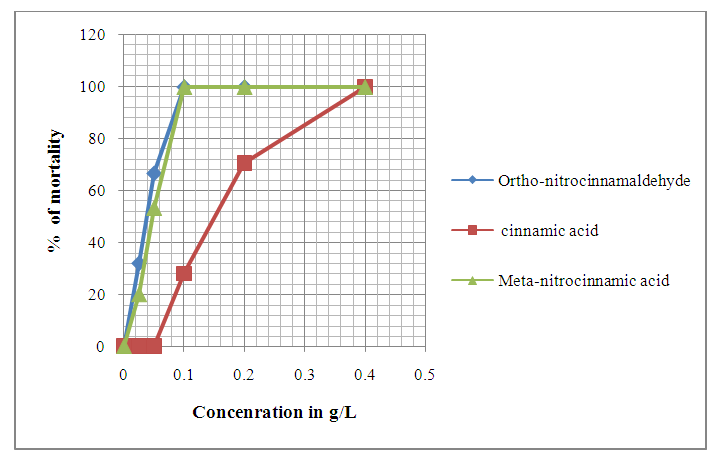 | Figure 9. Evolution of Anophèles gambiae eggs mortality after 72 hours of exposition to different concentrations of chemical compounds |
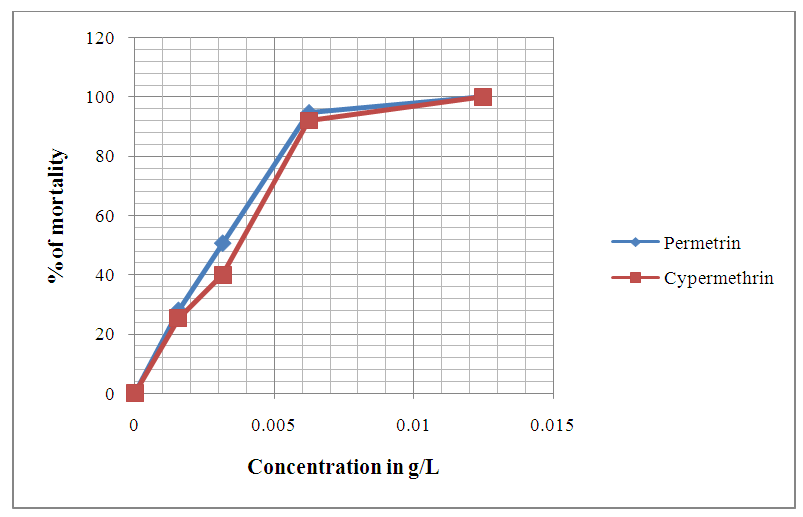 | Figure 10. Evolution of Anophèles gambiae eggs mortality after 72 hours of exposition to different concentrations of chemical compounds |
4. Conclusions
- Our research focuses on vector control in the fight against malaria. After careful examination of the chemical constituents of the essential oils studied, eleven compounds were targeted based on the outcome of our previous work, done on essential oils and their larvicidal and ovicidal activities. The evaluation of the larvicidal and ovicidal activities of these compounds, synthesized in our laboratory or obtained from trade has been carried out. Further studies will be carried out on the structure activity relationship of compounds containing conjugated double bound to functional group. The results showed that the chemical constituents of essential oils are more active than the corresponding essential oils. The activities observed corroborate with other published research works, which demonstrated that eggs were more sensitive than larvae at doses about 5 times lower. This work has shown that compounds as simple as cinnamic acid or cinnamaldehyde can also be exploited. These chemicals are industrially available and have little impact on the environment as they are potentially biodegradable.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML