-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2022; 12(5): 91-93
doi:10.5923/j.chemistry.20221205.02
Received: Nov. 1, 2022; Accepted: Nov. 19, 2022; Published: Dec. 28, 2022

The Mechanism of Nylander’s Test for Glucose in Urine
Francisco Sánchez-Viesca, Reina Gómez
Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico
Correspondence to: Francisco Sánchez-Viesca, Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The Nylander’s test for glucose detection in urine is based on the reduction of bismuth subnitrate to elemental bismuth in alkaline medium and in the presence a Seignette’s salt. However the reaction route and the mechanisms have not been advanced. In this communication both of them are provided, each step being fully commented. Since Nylander’s assay is a top test not surpassed in sensitivity by other method it is interesting know the series of steps from the reagents to the final elemental bismuth separation that occurs via a one electron transfer in a bimolecular reaction. That is, through the bismuth tartrate complex and several organometallic intermediates. This Theoretical Organic Chemistry Study is important since glucose is an analyte.
Keywords: Bismuth subnitrate, Glucose, Potassium sodium tartrate, Reaction mechanism, Reactive intermediates, Redox reactions
Cite this paper: Francisco Sánchez-Viesca, Reina Gómez, The Mechanism of Nylander’s Test for Glucose in Urine, American Journal of Chemistry, Vol. 12 No. 5, 2022, pp. 91-93. doi: 10.5923/j.chemistry.20221205.02.
1. Introduction
- The Nylander’s test is an optimum assay for glucose detection in urine not overtaken by any other method.The test is based on the reduction of bismuth subnitrate by glucose in alkaline medium in the presence of Rochelle salt. The reaction route has not been provided, and less the mechanism. In this communication we supply both of them in accordance with the experimental procedure since the addition order of the reagents is of utmost importance.This paper is a follow up of our studies on reaction mechanism [1-5].
2. Antecedents
- The test under study is due to Emil Nylander. He published his test in an analytical journal [6] and in a journal of physiological chemistry [7]. His test was recorded in Germany [8,9] and in the United States [10].The test is as follows: Dissolve 4 g of sodium tartrate in 100 ml of a 10% NaOH solution. Then add 2 g of bismuth subnitrate. Heat the mixture to 50°C and filter after cooling. Preserving the reagent, even for months, leaves it unaltered. Boil 10 ml of urine with 1 ml of the reagent for 2 min. When sugar is present there is a brown to black colouration of the fluid where metallic bismuth settles down. If the dark colouration occurs as the fluid is cooling down, it does not prove the presence of sugar. The reaction takes place when the proportion of sugar in the urine is 0.1%. If the test is negative there is no need to do any further test, [11]. Potassium sodium tartrate (Rochelle salt) is also used [12].Bismuth is a post transition metal having 83 electrons in six shells, [13]. The electron configuration of ground state gaseous neutral bismuth is: [Xe] 4f14 5d10 6s2 6p3, [14]. Thus bismuth is trivalent.
3. Disscusion
- The activation of the glucose molecule takes place in alkaline medium since this aldose is a carbon acid and forms a reactive enolate in basic conditions. On the other hand the reagent, bismuth subnitrate, forms insoluble bismuth hydroxide with sodium or potassium hydroxide, [15, 16]. In order to avoid this reaction and maintain bismuth in solution even in basic medium it must be taken as tartrate complex.The nitrate and one hydroxyl group in bismuth subnitrate are displaced by carboxylate and the vicinal alkoxide in tartrate anion. Besides the 5 member heterocyclic ring there remains a carboxylate for solubility, Figure 1.
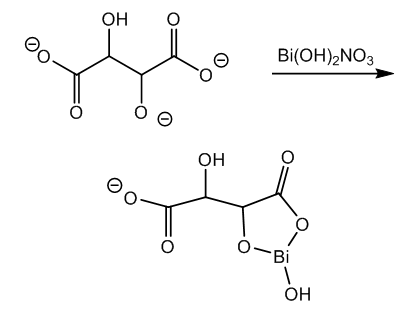 | Figure 1. Formation of bismuth tartrate complex |
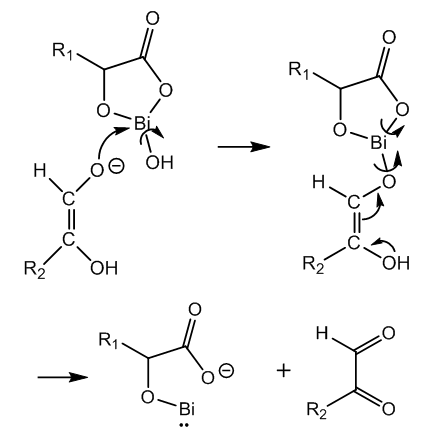 | Figure 2. First redox reaction to Bi-I complex and keto glucose |
 | Figure 3. Formation of one key intermediate |
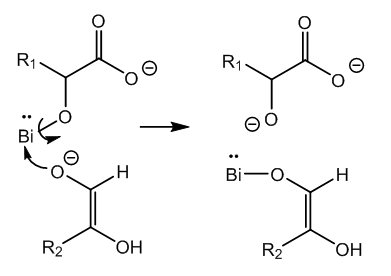
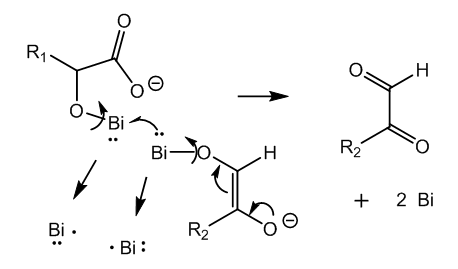 | Figure 4. Bimolecular reaction via one-electron transfer between two key intermediates |
4. Conclusions
- In Nylander’s test for glucose bismuth subnitrate in the presence of Rochelle salt is reduced by glucose in alkaline medium to elemental bismuth. This brief experimental account of a top test deserves the study of what is happening in the test tube.Bismuth must be as tartrate complex in order to avoid formation of insoluble hydroxide in alkaline medium. The latter is necessary to activate the glucose molecule. Several organometallic intermediates are formed. Finally a bimolecular reaction between two different bismuth-I intermediates produces two atoms of elemental bismuth via a one-electron transfer.Glucose is oxidized to keto-glucose (D-glucosone) and isomerized to gluconic acid by an internal Cannizzaro reaction. Three molecules of glucose and two molecules of bismuth tartrate complex produce two atoms of elemental bismuth and three molecules of keto-glucose.The present Theoretical Organic Chemistry Study has cleared out completely the reaction route and the mechanism of Nylander’s test for glucose in urine.
ACKNOWLEDGEMENTS
- Thanks are given to Martha Berros for support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML