-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2022; 12(5): 85-90
doi:10.5923/j.chemistry.20221205.01
Received: Nov. 1, 2022; Accepted: Nov. 19, 2022; Published: Nov. 24, 2022

Investigating Cooperativity Between Pd (0) and Sb (V) in Bis(2-Picolyl)-Pd-SbCl3 Using Lewis Bases: A Case Study Using DFT
Arjun C. Bhowmick1, 2, 3, Julia M. Fitzgerald1, Alexandra Z. Chafetz1
1Department of Chemistry and Biochemistry, Kalamazoo College, Kalamazoo, Michigan, USA
2Department of Chemistry, University of South Dakota, Vermillion, South Dakota, USA
3Department of Chemistry, Mawlana Bhashani Science and Technology University, Santosh, Tangail, Bangladesh
Correspondence to: Arjun C. Bhowmick, Department of Chemistry and Biochemistry, Kalamazoo College, Kalamazoo, Michigan, USA.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Model complexes 1-4 have been studied using the method density functional theory (DFT). The cooperativity between Pd-Sb is noted while binding Lewis bases. NBO analysis is applied to understand the metal contribution, electron occupancy in orbitals, and types of bonds formed in Pd-Sb. Although the theoretical study suggests that the bond is partially covalent in nature as we see the Wiberg bond order index 0.6 (model complex 2-4), the net Pd-Sb bonding interaction is repulsive. The study clearly illustrates the future development of 2-picolyl-based metal complexes and assesses their suitability as a catalyst.
Keywords: Lewis Acid, Lewis Base, Metal complex, DFT, NBO
Cite this paper: Arjun C. Bhowmick, Julia M. Fitzgerald, Alexandra Z. Chafetz, Investigating Cooperativity Between Pd (0) and Sb (V) in Bis(2-Picolyl)-Pd-SbCl3 Using Lewis Bases: A Case Study Using DFT, American Journal of Chemistry, Vol. 12 No. 5, 2022, pp. 85-90. doi: 10.5923/j.chemistry.20221205.01.
Article Outline
1. Introduction
- Metal complexes are being used as a catalyst over a long period as they are catalyzing various reactions involving organic synthesis [1-5]. The superiority of one metal complex over another plays a vital role while choosing metal complexes and in this continuous effort, researchers are developing metal complexes having low-cost earth-abundant metals to replace precious metals [6]. In the last couple of years, monometallic, homo-bimetallic, hetero-bimetallic, and multi-metallic complexes have been developed a lot and additionally, researchers successfully assessed their catalytic performance [7,8]. It was observed that the synergistic effect of the hetero-multi metallic system plays a vital role [9] over their mono or homo metallic systems. The recent discovery by Stephan and coworkers [10] of metal-free LA-LB (Lewis’s acid-Lewis’s base) pairs for activating H2 revolutionized a new pathway of research. Now, researchers are concentrating on LA-LB homo or heterobimetallic systems for activating small molecules [11,12] and synthesizing various value-added products in the development of metal catalysts [13-17]. The novelty of the method is that the nearby Lewis acidic metal center helps in catalyzing reactions over other metals taking some electron density and this is known as cooperativity [18-20]. Putting an electron-withdrawing group on a Lewis basic metal center or oxidizing it makes the Lewis base metal further electron deficient in nature. The Lewis basic metal now helps in withdrawing electron density from the nearest metal, which is the key concept in catalysis [21-25] by this LA-LB system. Here we are trying to assess the cooperativity between the Sb-Pd system for binding Lewis bases [26] using the method DFT. We choose a model complex bis(2-picolyl)-Pd(0)-Sb(v)-Cl3 (1) and it is not available in the literature. We believe the research may attract the experimental research groups to design and synthesize the complexes of similar or different systems by investigating their catalytic application, as we see from the theoretical study the complexes show a positive effect for cooperativity between two metals while binding Lewis bases.
2. Quantum Chemical Calculations
2.1. Computational Details
- We used density functional theory (DFT) to investigate the geometry and electronic structures of the complexes. The complexes were optimized in gaussian 16. M06-L functional [27] was selected with def2-TZVP [28,29] basis set for Pd and Sb and def2-SVP [30] was used for C, H, N, P, and Cl. All calculations were done in the solvent phase using the method PCM [31] and the solvent was used tetrahydrofuran. Molecular orbitals were drawn by the chem craft software (free version) choosing isosurface 0.03. Harmonic vibrational analysis was performed at the same level to verify the nature of the stationary point. In addition, NBO analysis was done using the same basis set and functionals as stated above for localizing the orbitals of interest [32].
3. Results and Discussions
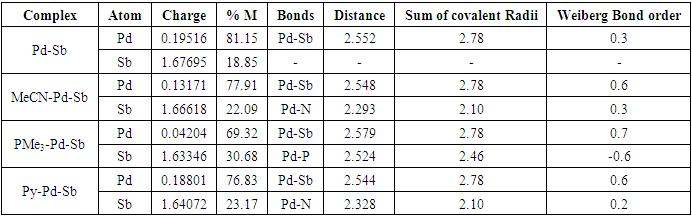
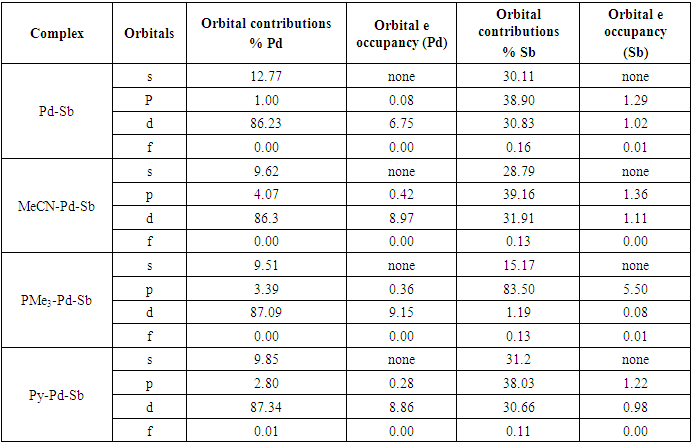
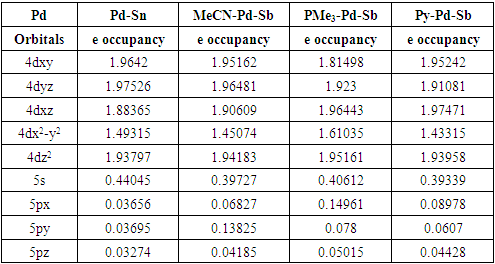
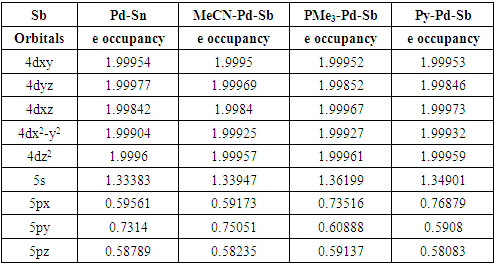
- The theoretical study (DFT) suggests that the singlet is the lowest energy ground state for model complex 1. The singlet and triplet energies are found at 0 kcalmol-1 and -10.7 kcalmol-1. The model complex bis(2-picolyl)-Pd(0)-Sb(V)-Cl3 (1) is shown in Figure 1. The oxidation state of the palladium is zero and the oxidation state of Sb is +5. We hypothesize that the electron-filled d orbital of Pd should interact with Sb empty orbital and transfer some electron density to Sb. In this way, Sb will act as a Lewis basic center. When Pd will donate electron density, the Pd center will be deficient in an electron, and it should now act as Lewis acidic in nature for incoming Lewis bases. Pd should have empty orbitals (5s, 5P) to accept any electron density from Lewis bases in close contact with Pd.
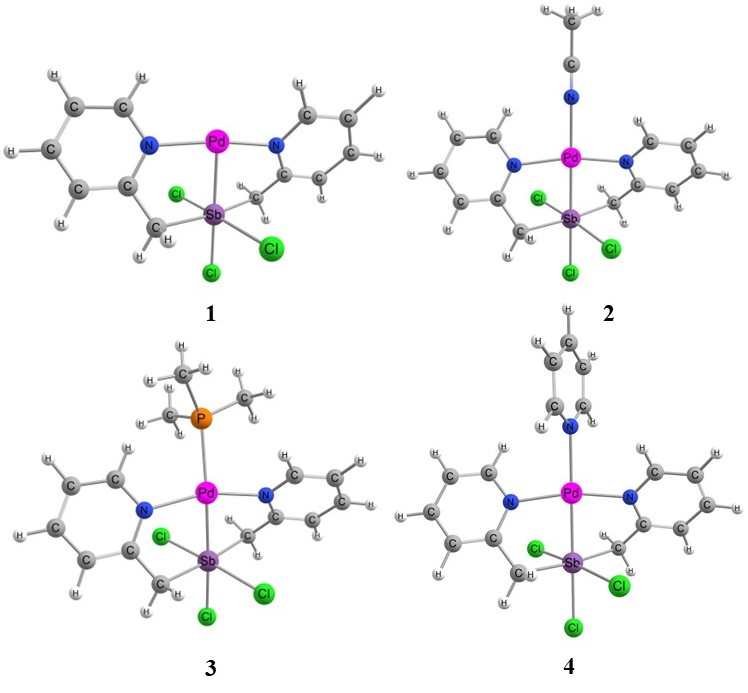 | Figure 1. The structure of model complexes |
|
|
|
|
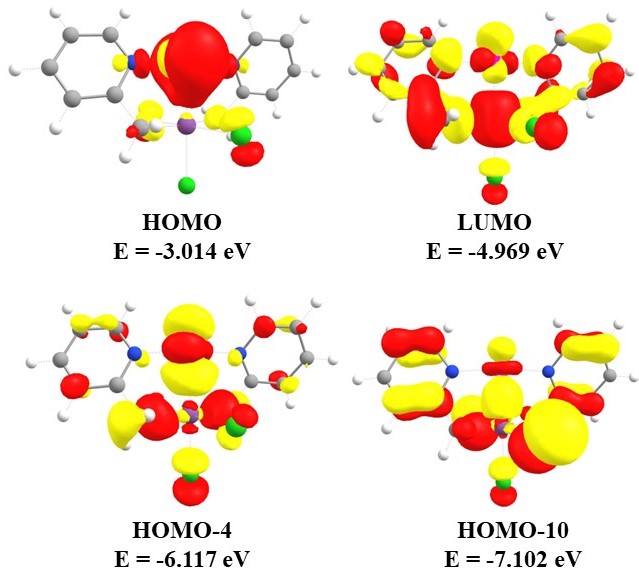 | Figure 2. Molecular orbitals of bis(2-picolyl)-Pd(0)-Sb(v)Cl3 (1) |
4. Conclusions
- In conclusion, we say that the model bimetallic bis(2-picolyl)-Pd(0)-Sb(v)-Cl3 complex shows some agreement with our hypothesis. We can say that the complex may be suitable as a catalyst in organic synthesis as the theoretical study suggests an electronic communication exists while binding Lewis bases. The Pd is playing a dual role such as Lewis’s base and Lewis acidic center while binding Lewis bases and the nearest Sb is acting as Lewis's basic center. Additionally, NBO analysis predicts that the interaction between Pd-Sb is partially covalent while interacting with Lewis bases. We believe our findings will be helpful to develop and tune better catalysts in the future.
ACKNOWLEDGMENTS
- We are thankful to the Super Computing systems at the University of South Dakota for allowing us to conduct this research using the supercomputer. I am also grateful to Dr. George Barroso for showing me the NBO analysis.
Conflicts of Interest
- The authors declare no competing financial interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML