-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2022; 12(4): 73-75
doi:10.5923/j.chemistry.20221204.02
Received: Aug. 26, 2022; Accepted: Sep. 16, 2022; Published: Sep. 24, 2022

The Mechanism of the Morphine-Apomorphine Rearrangement
Francisco Sánchez-Viesca, Reina Gómez
Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico
Correspondence to: Francisco Sánchez-Viesca, Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Apomorphine is obtained from morphine by cationic rearrangement of morphine and is used in the diagnosis and treatment of Parkinson’s disease and also in erectile dysfunction. A reaction mechanism for apomorphine formation was suggested 60 years ago. In this communication a direct and well supported mechanism is given. Acid catalyzed dehydration of the allylic alcohol in the morphine molecule causes double bond migration and formation of an allylic carbonium ion at C-8. This ion is the driving force for a 1,3-migration by C-C breaking of the ethanamine chain of the piperidine ring, eliminating the three dimensional overhanging bridge, forming an almost flat new D-ring. A more stable carbonium ion is formed at C-13 which is neutralized by deprotonation giving a double bond conjugated with the benzene ring. Protonation of the dihydrofuran ring forms an oxonium ion and fission at the side of the cyclohexadiene ring. Neutralization of the carbonium ion by double bond migration and deprotonation affords the second benzene ring in apomorphine. The old mechanism is discarded since it includes a step forbidden by thermodynamics.
Keywords: Apomorphine, Electron dynamics, Morphine, Reactive intermediates, Stereochemistry, Thermodynamics
Cite this paper: Francisco Sánchez-Viesca, Reina Gómez, The Mechanism of the Morphine-Apomorphine Rearrangement, American Journal of Chemistry, Vol. 12 No. 4, 2022, pp. 73-75. doi: 10.5923/j.chemistry.20221204.02.
1. Introduction
- Apomorphine is synthetic derivative of morphine (απó, from) and it is sold as expensive brand medicine, Figure 1.
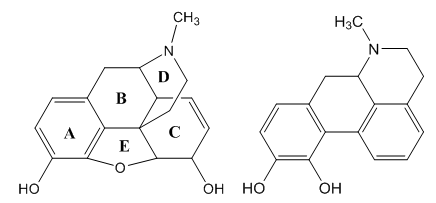 | Figure 1. Morphine and apomorphine molecules |
2. Antecedents
- Arpe dissolved morphine in an excess of sulphuric acid and the acidic liquid was slid off until decomposition begins. The white body separated by water addition contains no morphine. This is the first obtention of apomorphine, [10].Apomorphine has been prepared by heating morphine with 10 times the weight of 35% HCl for two to three hours at 140° to 150°. The base is precipitated with sodium bicarbonate, extracted with ether, and crystallized as the hydrochloride on addition of concentrated HCl to the ether. [11].A sensitive test for apomorphine was described by Grimbert and Leclère: a solution of apomorphine salt is boiled a few seconds with 5 drops of saturated HgCl2 solution and 5 drops of 10% sodium acetate solution, and extracted with amyl alcohol, the latter is coloured blue; sensitive to 1 part in 500,000. If much apomorphine is present, a blue precipitate is formed, which is soluble in amyl alcohol, [11].Apomorphine is prepared by heating morphine (1.00 g) in phosphoric acid (5.01 g) in the presence of phosphorus pentoxide (0.48 g) as water scavenger, heating at 90-100°C under an inert atmosphere. The resulting solution was stirred at that temperature for 2 hours to provide crude apomorphine in 63% yield. Pure apomorphine hydrochloride was obtained after hydrolysis of the phosphate, pH adjusting to liberate the base, extraction and conversion into the hydrochloride, [12].A Hungarian method uses methansulphonic acid for the microwave assisted rearrangement of morphine to apomorphine, heating at 90°C during five minutes. Yield, 73%, [13].A reaction mechanism for this transformation was advanced by Bentley in 1965, Figure 2, [14]. Bryant repeated it in 1987 [15], and appeared in the 2014 edition of Bentley’s book [16].
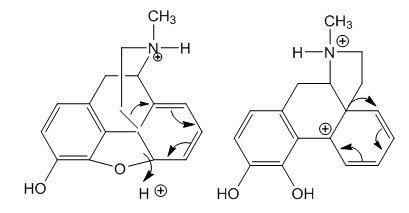 | Figure 2. Bentley’s sketch for apomorphine formation. Two key intermediates are missing |
3. Discussion
- In the present Theoretical Organic Chemistry study we provide a direct and sustained mechanism. It is based on the chemical deportment of the functional groups present in morphine, is in accordance with the reaction medium, and is supported by the dynamics of electrons in organic reactions, the stereochemistry and by thermodynamics. The old mechanism is discarded as indicated for wards.The morphine-apomorphine rearrangement starts by the acid promoted dehydration of the allylic alcohol at C-6, Figure 3. Double bond shift generates an allylic carbonium ion at C-8. This carbocation is the driving force for a 1,3-migration via ring opening of the bridged piperidine ring. This structural reorganization shift removes the three dimension ethanamine chain forming now an almost planar new D-ring, that is, the expected fused ring. There has been steric improvement.
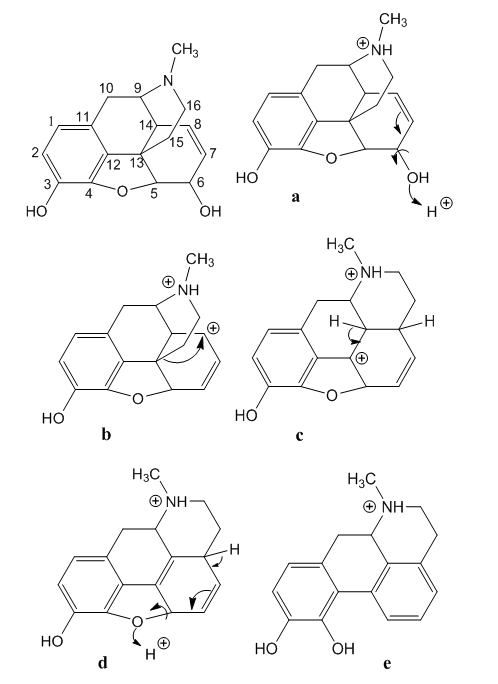 | Figure 3. The supported mechanism of the morphine-apomorphine rearrangement |
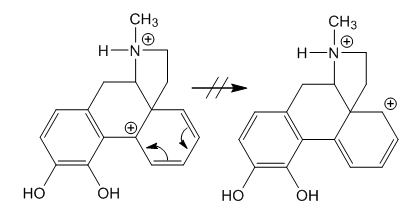 | Figure 4. This step is forbidden by thermodynamics |
4. Conclusions
- A new reaction mechanism for the morphine apomorphine rearrangement is provided. After dehydration of the allylic alcohol in morphine a carbonium ion at C-8 is formed which was overlooked in the old mechanism. This reactive intermediate is very important since C-8 is the position for ring closure of the new ring D, and the carbonium ion is the driving force for a 1,3-migration that eliminates the bulky bridge of the piperidine ring in favour of an almost planar new D-ring. The carbonium ion formed after migration is more stable than the previous one, that is, tertiary, benzylic instead of secondary, allylic. Moreover, it is more distant from the positive charge at nitrogen. The rest of the rearrangement is described in the “Discussion”.In the old mechanism there is a detour involving contraction to a five-member ring followed by expansion again through a step that is forbidden by thermodynamics. Besides, by this way there would be to C-C cleavages instead of one. Thus that route is discarded.
ACKNOWLEDGEMENTS
- Tanks are given to Martha Berros for support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML