-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2022; 12(4): 65-72
doi:10.5923/j.chemistry.20221204.01
Received: Aug. 24, 2022; Accepted: Sep. 2, 2022; Published: Sep. 15, 2022

Optimization of Combined Catalytic Ethanolysis of Ceiba pentandra (L.) Seed Oil and Analysis of Metal Trace Elements in Produced Biodiesel
Papin Sourou Montcho1, 2, 3, Sako Alphonse Avocefohoun1, Euloge Sènan Adjou1, Alassane Youssao Abdou Karim1, Cokou Pascal Agbangnan Dossa1, Anna Chrostowska3, David Bessieres2, Félicien Avlessi1, Codjo Koko Dominique Sohounhloue1
1Laboratory of Study and Research in Applied Chemistry, Polytechnic School of Abomey-Calavi, University of Abomey-Calavi (LERCA / EPAC / UAC), Cotonou, Benin
2Laboratory of Complex Fluids and their Reservoirs, Multidisciplinary Institute for Applied Reseach in Petroleum Engineering, University of Pau and Pays of l'Adour (LFCR / IPRA / UMR 5150 / E2S UPPA), PAU, France
3Institute of Analytical and Physicochemical Sciences for the Environment and Materials, University of Pau and Pays of l'Adour (IPREM / UMR 5254 / E2S UPPA), Pau, France
Correspondence to: Codjo Koko Dominique Sohounhloue, Laboratory of Study and Research in Applied Chemistry, Polytechnic School of Abomey-Calavi, University of Abomey-Calavi (LERCA / EPAC / UAC), Cotonou, Benin.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Ethanolysis of seeds oil from Ceiba pentandra with ethanol in combined catalysis was investigated in this study. In addition, the metal trace elements (TMEs) of the formulated biodiesel were quantified. For this purpose, the formulation of ethyl esters was done in two steps (acidic and basic) followed by purification. The influence of the molar ratio of ethanol to oil (6:1-8:1), the ratio of the mass of catalyst to oil (1.0; 1.1; 1.25 and 1.5) and the reaction temperature (30-60)°C were studied in order to optimize the operating conditions of this reaction. Subsequently, part of oil was converted to ethyl esters with the optimal reaction parameters. The analysis of metal trace elements using inductively coupled plasma mass spectrometry (ICP-CRC-MS, Agilent 7500) lead to determine the metal trace elements in both samples (oil and biodiesel) after mineralization. Calibration was performed with aqueous standards to avoid the use of organic standards which are often unstable. Fatty acids in the oil were converted into ethyl esters under optimal conditions (ethanol/oil molar ratio of (40:1), the amount of catalyst (H2SO4) (10% w/w) and a temperature of 78°C) for a fatty acid content equal to 0.46% (w/w). Triglycerides in oil were transesterified under basic catalysis with the optimal operating conditions (ethanol/oil molar ratio = 6:1, amount of catalyst = 1.1% (w/w) and reaction temperature = 60°C) yielded of improved ethyl esters equal to 98.91% (w/w). The MTEs of the vegetable oil samples and the formulated biodiesel revealed relatively low concentrations. On the other hand, other TMEs (Mg, Na, K and Ca) were quantified with concentrations that are higher (sup 0.1 µg/Kg) but lower than the recommended value (<500 µg/Kg) (ASTM D6751/ EN 14214). Data recovery was in the range of 90 to 100% for the two samples studied.
Keywords: Ethanolysis, Combined catalysis, Metal trace elements, Vegetable oil, Formulated biodiesel, Ceiba pentandra, ICP-MS
Cite this paper: Papin Sourou Montcho, Sako Alphonse Avocefohoun, Euloge Sènan Adjou, Alassane Youssao Abdou Karim, Cokou Pascal Agbangnan Dossa, Anna Chrostowska, David Bessieres, Félicien Avlessi, Codjo Koko Dominique Sohounhloue, Optimization of Combined Catalytic Ethanolysis of Ceiba pentandra (L.) Seed Oil and Analysis of Metal Trace Elements in Produced Biodiesel, American Journal of Chemistry, Vol. 12 No. 4, 2022, pp. 65-72. doi: 10.5923/j.chemistry.20221204.01.
Article Outline
1. Introduction
- Concern about the disappearance and gradual depletion of the world's fossil fuel reserves has prompted scientifics to research and produce alternative renewable fuels [1]. Curiosity about green fuels as an additive or alternative to petroleum has grown considerably over the past two decades for political, financial and environmental reasons [2,3]. Thus, several developed fund have supported researches for more environmentally friendly and renewable resources in order to reduce climate change. One of options was the production of biodiesel. Indeed, biodiesel is a mixture of alkyl esters obtained by the transesterification reaction from vegetable oils. In order to respond effectively to food self-sufficiency, the use of non-edible vegetable oils for the formulation of biodiesel is nowadays promoted. It is shown that the use of biodiesel allows the reduction of carbon dioxide (CO2) emissions [4]. Ceiba pentandra (L.) known as Kapokier is a tropical plant which belong to the family of Bombacaceae. Kapokier is known for its soft and white silk [5]. It is a large tree with conical thorns on the trunk and twigs having a rapid growth and becomes productive between 4 to 5 years. Under optimal conditions an adult tree can produce up to 400 fruits each year, yielding 15-18 kilograms of fiber and about 30 kilograms of seeds per fruiting [6]. Fruit is in a spindle-shaped membranous envelope containing black spherical seeds representing about 28% m/m of the fruit and covered with fine, silky hairs. Seeds are a source of oil and therefore an excellent raw material in the paint and biodiesel production industries [7,8]. Previous study reported that the main constituents of ethyl biodiesel derived from Ceiba pentandra vegetable oil are palmitate (C16:0) (24.53%), oleate (C18:1) (19.6%) and linoleate (C18:2) (36.92%) esters [8]. Oils in addition to the main elements such as carbon (82-87%) and hydrogen (12-15%) [9]; contained trace metals such as sulfur (0.05-5%), oxygen (< 2%) and nitrogen (< 0.1%) which came respectively in fourth and fifth position of the most abundant elements [10]. Other elements such as vanadium, nickel, calcium, potassium, iron, copper, zinc, boron, arsenic, selenium, silicon, phosphorus are generally present in oils in small proportions (0.01-0.1%) or in ultra trace levels (<0.01%). Indeed, trace metal elements (TMEs), are incisive pollutants that have a toxic and harmful impact on alkyl esters (biodiesels) and their use. Among these TMEs, Lead, Zinc, Copper, Potassium, Sulfur, Sodium have an important place. Their presence and accumulation in oil and biodiesel have several origins including, the supply and misuse of fertilizers based on these elements. In addition, TMEs play an important role in the clogging of vehicle fuel lines and can lead to the production of undesirable residues of metal oxides in engine. Moreover, some TMEs acted as poisons during the catalytic cracking process of oils in the refining process, particularly vanadium and nickel, which can occur at high concentrations [11]. Other metal trace elements can cause corrosion of refinery equipment [12]. Mercury can be deposited in engine equipment, requiring careful monitoring and maintenance [13]. Therefore, the determination of trace metal elements in seeds oils with could lead to, know the type of specific metals which should be removed upstream and downstream [14]. On the other hand, some heavy metals are toxic at high doses. Thus, monitoring of these elements (Ca, Cu, Fe, Mg, Mn, Na and P etc.) is necessary because of their ability to affect the behavior of biodiesel by forming undesirable compounds in engine. Then, the present study aims to optimize the combined catalytic transesterification reaction of non-edible seed oil from Ceiba pentandra and quantify metal trace elements in seeds oil and biodiesel produced.
2. Material and Methods
2.1. Sampling and Oil Extraction
- Ceiba pentandra seeds were collected in northwestern Benin 9° 39′ 42″ North then 1° 23′05″ East. Oil was extracted from seeds soxhlet extraction method and stored in dark at 25°C until use [5,8].
2.2. Transesterification Process
- Biodiesel was produced by the transesterification reaction in combined catalysis (acid and basic) from non-conventional oil obtained. Ethanolysis reactions were carried out, following the two-step procedure (combined acid and base homogeneous catalysis), considering the acidity higher than 1% (m/m) oil [5].
2.3. Fischer's Esterification
- The first step consisted of esterification of free fatty acids from oil by homogeneous acid catalysis. It was conducted with anhydrous ethanol: oil molar ratios of (9:1, 14:1, 20:1 and 30:1) in the presence of concentrated sulfuric acid (H2SO4) (1 to 5% (w/w oil) at 78°C for one hour under moderate stirring.At the end of this step, the reaction mixture was neutralized with 20% (w/w oil) at 2% (w/w water) sodium bicarbonate solution for 10 min under slow stirring, and then transferred to a separatory funnel. To accelerate the separation of the 100% (v/v) phases, hexane was added to the mixture. After phase separation, drying was performed by addition anhydrous sodium sulfate (Na2SO4). After filtration the ester phase, the solvent was evaporated under reduced pressure. The ester-rich phase was then weighed and free fatty acid content was determined by colorimetric titration.Transesterification of triglycerides by homogeneous basic catalysis.Several experiments of the synthesis of ethyl esters by the transesterification reaction were carried out to evaluate the stoichiometric amount of ethanol to be used. For this purpose, 5g of non-conventional seeds oil were transesterified by varying the amount of ethanol to analyze the effect of the molar ratio (ethanol: oil) on the reaction. Indeed, 6:1 and 8:1 ethanol excesses were used to transesterify oil samples [15].
2.4. General Process in Basic Homogeneous Catalysis
- Unreacted triglycerides and mono- and di-glycerides contained in the ester-rich phase were transesterified under basic catalysis in second step. Ethanol and catalyst were added in the amounts established for each experiment, the stirring system was connected (250 rpm) and this time represented the start of the reaction. The final mixture was neutralized with a 3% NaHCO3 solution (w/w water) to pH=7 and then transferred to a separatory funnel. After separation, the ester phase was then dried with anhydrous sodium sulfate (Na2SO4) and filtered through filter paper. Residual ethanol and water were removed by evaporation at 90°C under reduced pressure. The yields of ethyl biodiesel from each oil were evaluated and expressed as mass percentage [15].
2.5. Trace Metal Analysis by Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
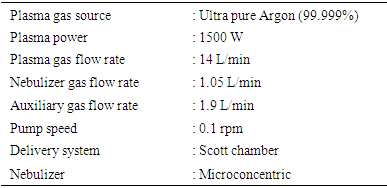
- Inductively coupled plasma mass spectrometry (ICP-CRC-MS, Agilent 7500) with a CRC-2 collision-reaction cell was used to determine the inorganic constituents in the seeds oils and biodiesels formulated after mineralization. The system (7500cs) equipped with a simultaneous dynode, discrete dual mode (pulse/analog) detector with nine orders of linear dynamic range uses a Scott double pass chamber and a low flow efficient microconcentric nebulizer and spray cooled Scott chambers. The ICP-MS is controlled by an Agilent Chemstation computer system. For sample preparation and mineralization, 200 µL of sample was added to 1.5 mL of oxygenated water (H2O2) and 2.5 mL of 65% Suprapur® nitric acid (HNO3) (Ref. 1.00441) in a 50 mL tube. Seeds oils are complex mixtures of organic materials, with a high organic load and viscosity, which can cause polyatomic and spectrometric interferences during the determination, and instability or even plasma quenching. Therefore, a pre-treatment is necessary before their introduction in the analytical instruments. Thus, the mineralization of the sample was done in a Digiprep system for 8h at 80°C.
|
3. Results and Discussion
3.1. Transformation of Free Fatty Acids into Ethyl Esters by Fischer Esterification in Acid Catalysis
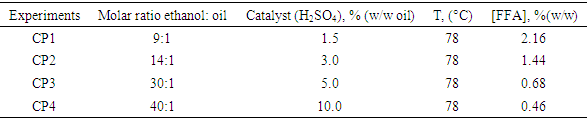
- Table 2 presented the results of transformation of free fatty acids into ethyl esters by Fischer esterification reaction. For the various tests carried out, a decrease in free fatty acid content is noted as a function of the anhydrous ethanol/oil molar ratio and the concentration of the catalyst. Thus, for a molar ratio (anhydrous ethanol / oil) and a concentration of catalyst (sulfuric acid) respectively (40:1) and 10% (m/m) of the mass of vegetable oil used, it was observed a better reduction of the free fatty acid content.
|
3.2. Transesterification of Vegetable Oil by Basic Catalysis
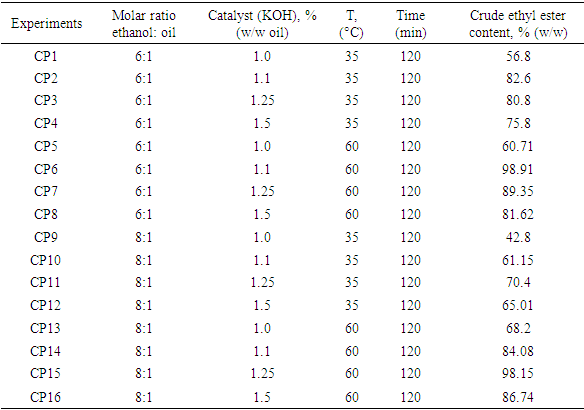
- Table 3 showed the yields of ethyl esters from ethanolysis under basic catalysis. The results obtained indicate that parameters such as molar ratio, amount of catalyst and temperature greatly influence the yield of oil conversion to esters. Thus, the variation of these factors allowed to optimize, the ethanolysis in basic catalysis. Subsequently, the different factors that influence this conversion were analyzed and a mode of ethanolysis by basic homogeneous catalysis was improved.
|
3.3. Influence of Ethanol: Oil Molar Ratios (6:1 and 8:1) on Yield at Ethyl Esters
- The experimental results of ethanolysis of oil (Figure 1) indicated that when the amount of ethanol is increased, an improvement in yield is observed. At 35°C and maintaining cKOH=1.1% of the oil mass, a conversion rate of 82.6% (w/w) was obtained in 120 minutes for an ethanol:oil molar ratio of 6:1, while for the same reaction time, we found an ester yield of 61.15% (w/w) by increasing the ethanol:oil molar ratio to 8:1. The same findings were made by performing the reaction under the same operating conditions with an increase in temperature to 60°C but with an improvement in the conversion rate (ω) in both cases (6:1; ω=98.91% (w/w) and 8:1; ω = 84.08% (w/w)). This result, shows the positive impact of temperature on the basic catalysis ethanolysis of C. pentandra seeds oil.
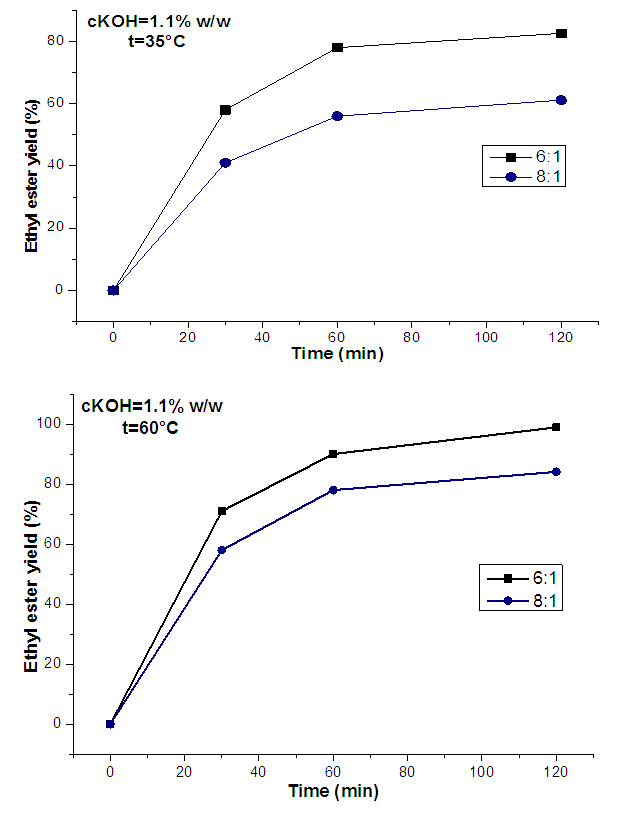 | Figure 1. Effect of molar ratio (6:1 and 8:1) on the yield of at ethyl esters from C. pentandra seeds oil with cKOH=1.10% at 35 and 60°C |
3.4. Influence of Catalyst Concentration (cKOH) on Yield at Ethyl Esters
- Figure 2 presented the results of the effect of catalyst con-centration (cKOH) on C. pentandra seeds oil conversion rate as a function of reaction time: ethanol:oil molar ratios (6:1 and 8:1) and temperature. The results of these tests have made it possible to specify the effect of amount of KOH and temperature on the rate of conversion of this oil into ethyl esters Indeed, the tests gave a better conversion rate with cKOH equal to 1.10%. But with higher cKOH (1.25 and 1.5%) or lower (1%), an incomplete reaction and or the appearance of secondary reaction (saponification) leading to a low yield of ethyl esters were observed. The highest yield of esters or conversion rate is 98.91% (w/w) after 120 minutes of reac-tion at 60°C with an ethanol: oil molar ratio of 6: 1 and 98.15% (w/w) under the same conditions but with a molar ratio of 8: 1. Indeed, the graphs show a high yield of esters from the first 60 minutes of reaction and then asymptotic curves until the end of reaction (120 min).
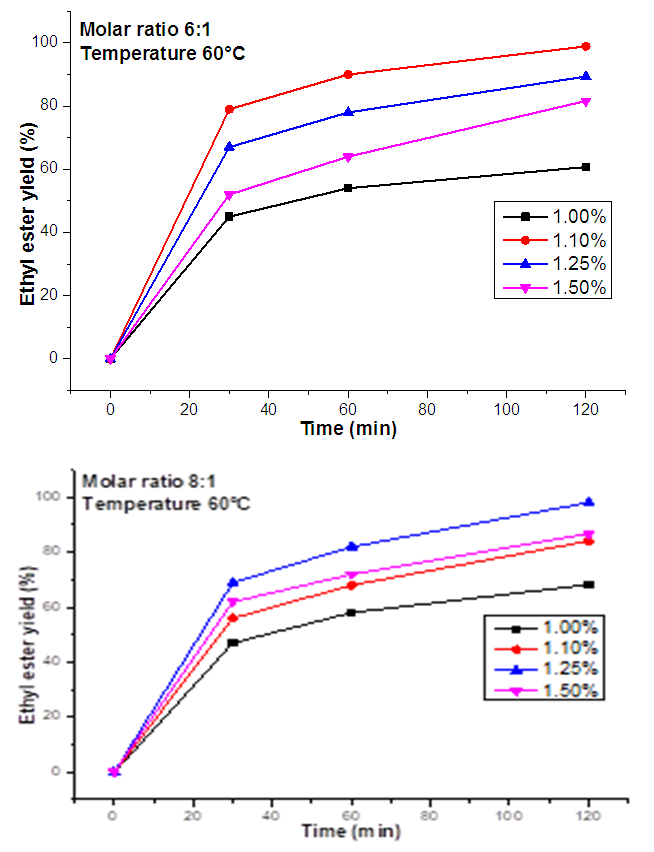 | Figure 2. Effect of catalyst concentration (cKOH) on C. pentandra seeds oil conversion rate as a function of reaction time: ethanol:oil molar ratios (6:1 and 8:1) and temperature |
3.5. Influence of Temperature on Ester Yield
- Figure 3 presented results of the influence of temperature on yield of esters from C. pentandra seeds oil: Ethanol: oil molar ratios (6:1; 8:1), cKOH=1.1% (m/m). Results showed the variation of the conversion rate to esters of C. pentandra oil as a function of time. In these experimentations, it was observed an improvement in the rate of conversion to esters, when reaction is conducted at 60°C. These are 98.91 and 98.15% (w/w) of conversion for molar ratios of 6:1 and 8:1 respectively. This finding, allows to say that the ethanolysis of C. pentandra oil is very efficient at a reaction temperature of 60°C than at 35°C. These results lead to define the optimal operating conditions for ethanolysis under basic catalysis after the transformation of free fatty acids into ethyl esters by the Fischer esterification reaction (Table 1). It appears that the increase in temperature allowed chemical equilib-rium to be reached faster by reducing reaction time. This behavior can be explained by exothermicity of transesteri-fication reaction by basic homogeneous catalysis as demonstrated by Nitièma-Yefanova and al. [18] during ethanolysis of seeds oils from Balanites aegyptiacas, Azadirachta india, and Jatropha curcas.
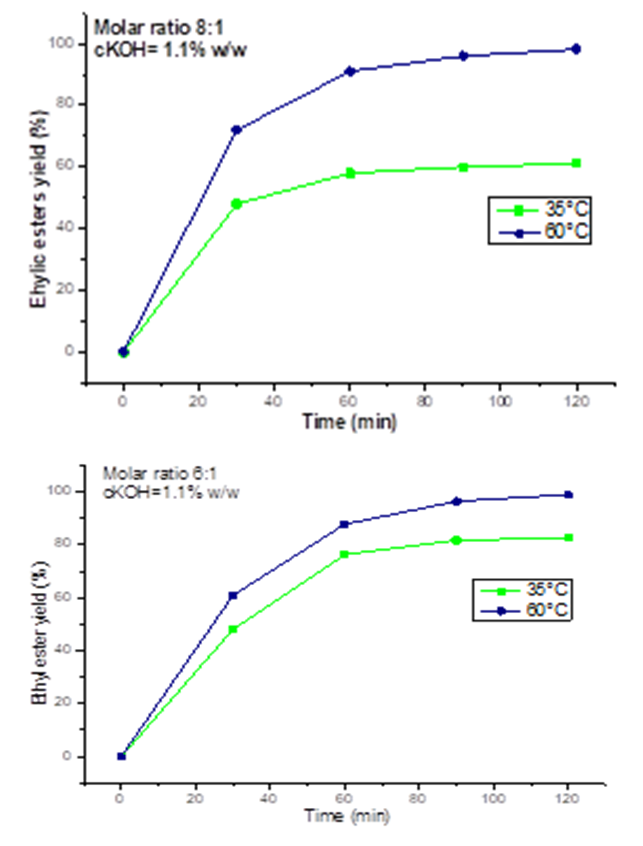 | Figure 3. Influence of temperature on yield of esters from C. pentandra seeds oil: Ethanol: oil molar ratios (6:1; 8:1), cKOH=1.1% (m/m) |
3.6. Trace Metals Elements (TMEs) in Vegetable Oil
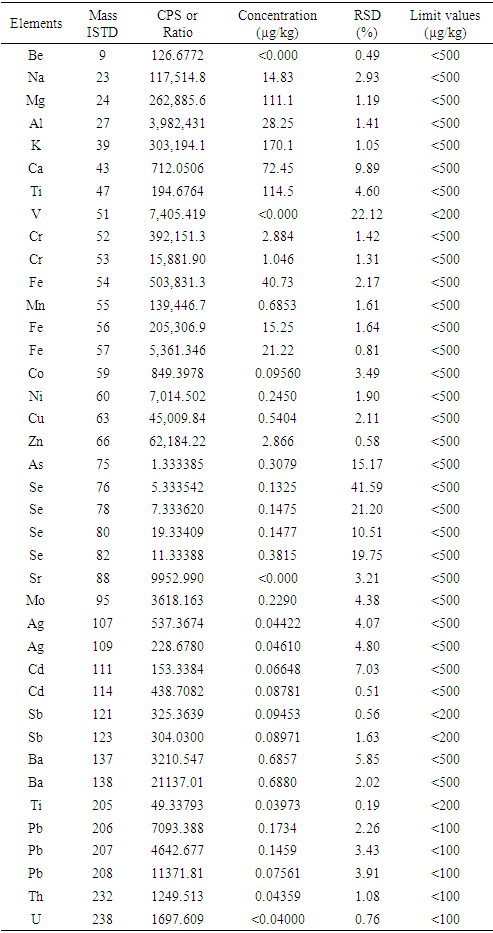
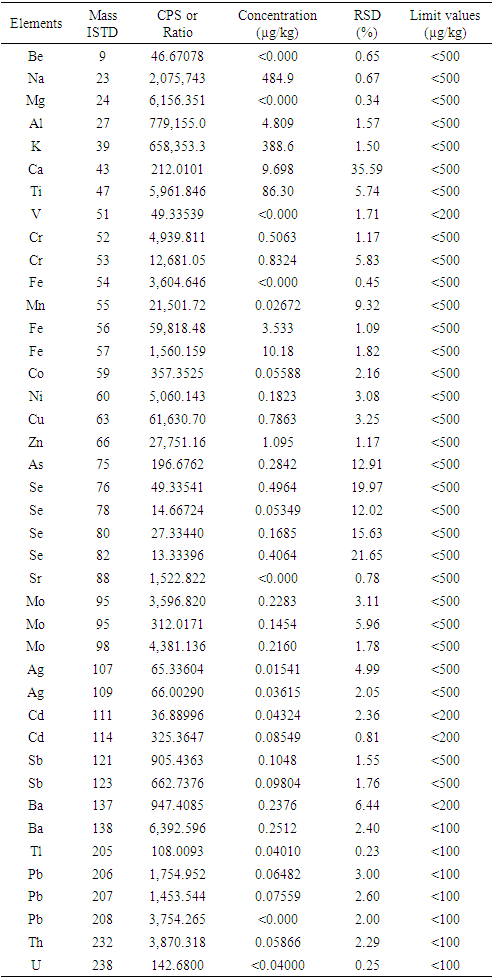
- The results of analysis of TMEs in seed oil performed by inductively coupled plasma mass spectrometry (ICP-MS) are presented in Table 4. Low values of concentration of analyzed minerals, such as Na, Mg, k, Ca, Ti, Mn, Zn and Fe, were obtained, when compared to those reported by Hossain et al. [19] This variation could be due to the edaphic conditions and the chemical composition of the investigated seed oil. On the other hand, some metal trace elements such as sodium, potassium, magnesium could be naturally present in this oil or introduced by pollution during chemical reactions with seed oil. However, it should be noted that all the contaminants quantified give lower values (<500 µg/Kg) as recommended by the ISO Standard (22241-2). However, the presence of TMEs such as Strontium, Selenium, Molybdenum, Silver, Barium and Lead in low concentrations (< 0.0001 µg/kg) in the seed oil is detected. It should be noted that the presence of TMEs (lead, cadmium) in seed oil at very low concentrations can be eliminated by the development of operating conditions of refining. Thus, the establishment of a quantification plan of these trace elements is necessary to have a reliable database to comply with current standards. Overall, the quantification of trace elements has allowed the evaluation of contaminants in seed oil. Moreover, the knowledge of these trace elements in the seed oil is necessary to avoid the formation of undesirable products during the transesterification reaction in basic catalysis.
|
3.7. Contaminants in Biodiesel from Ceiba pentandra Seeds Oil
- The results of the analysis of contaminants in biodiesel derived from seed oil of Ceiba pentandra are presented in Table 5 produced contains 14.83 µg/kg of sodium (Na) and 170.1 µg/kg of potassium (K). It should be noted that these trace metals (sodium and potassium) are residues of the catalysts (NaOH or KOH) and must be removed during the washing of the final product. Other elements such as Iron, Nickel, Copper, Zinc, Barium are also quantified but in smaller quantities. The values of the analyzed minerals are all lower than the ISO standard (22241-2) fixed at <500 µg/kg for biofuels. Thus, the analysis of biodiesel formulated by ICP/MS shows a decreasing concentration in this order: Na > K > Ti > Ca > Zn > Fe = Cu > Cr = Se > Ni = As = Ba > Ag > Sr > Co > Cd (Table 4). However, it is important to note that these inorganic elements may be present in the feedstock or introduced during the biodiesel production processes. Previous study has reported that they can occur during base-catalyzed ethanolysis and storage of formulated biodiesel or added to the fuel as an additive to improve diesel engine performance [20,21]. However, some study indicate that the main source of metals in biodiesel is the feedstock used [22]. In addition, the content of TMEs can be related to air and soil pollution. The main conclusion of this work is that a large number of elements were found, but with very low concentrations and in accordance with the specifications of the fuels.
|
3.8. Comparison of TMEs in Seed Oil and Biodiesel Produced
- Contaminants found in the seed oil such as Na, Mg, Al, K and Ca are at lower concentrations than those found in the formulated biodiesel. This increase of the contaminants in the formulated biodiesel should be due to the catalysts (KOH and NaOH) used during ethanolysis. The concentrations and responses obtained in number of courses per second show linear relationships. The regression coefficients are all greater than 0.99 for each trace metal element.
4. Conclusions
- This work is part of optimization of operating conditions such as molar ratio ethanol: oil, amount of catalyst and temperature in order to improve yield of biodiesel by com-bined catalysis on one hand and on other hand, quantifi-cation of trace metal elements (TMEs) present or introduced in the seed oil and formulated biodiesel. The experiments of ethanolysis of oil from Ceiba pentandra seeds allowed to identify the optimal operating conditions to reach the maximum conversion rate to biodiesel. Thus, a yield of 98.91% (m/m) of ethyl esters was achieved at optimal op-erating conditions (ethanol:oil molar ratio of 6:1, tempera-ture 60°C and a quantity of catalyst depending on acidity of oil used).. Moreover, identification of TMEs in oil of C. pentandra is important to understand their formation and their impacts in proper functioning of diesel engines and on environment. In addition, it should be noted that most of the trace metals are naturally present in this oil, but others are introduced into biodiesel formulated during the basic cata-lytic ethanolysis processes.
ACKNOWLEDGEMENTS
- Authors are grateful to French government via Cooperation and Cultural Action Service (SCAC) near Benin (Cotonou) for the scholarship. This work was carried out as part of a doctoral thesis jointly supervised by University of Pau and the Pays of l'Adour (France) and University of Abomey-Calavi (Benin).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML