-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2022; 12(2): 32-39
doi:10.5923/j.chemistry.20221202.02
Received: Mar. 1, 2022; Accepted: Mar. 30, 2022; Published: Apr. 15, 2022

Geopolymers Filters from Calcined Clay and Sugarcane Bagasse Blended with Activated Carbon for Removal of Selected Heavy Metals from Wastewater
D. M. Mwaura1, H. M. Mbuvi1, F. M. Maingi2
1Department of Chemistry, Kenyatta University, Kenya
2Department of Science Technology and Engineering, Kibabii University, Kenya
Correspondence to: F. M. Maingi, Department of Science Technology and Engineering, Kibabii University, Kenya.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The growth of industries and the exploitation of natural resources have led to increase of heavy metals concentrations in the earth’s surface, atmosphere and water bodies. Heavy metals pose serious environmental and health effects in the society since they are toxic and non-biodegradable. Removing heavy metals from aqueous solutions especially wastewater has become an area of study in the recent past. Various methods like ion exchange, reverse osmosis, coagulation and adsorption among others have been put forth in the removal of heavy metal ions. Geopolymers are zeolite analogues and there is little information on their use as water filters in literature. Incorporation of geopolymers and activated carbon to form wastewater filters have not been studied. This study focused on fabricating geopolymer filters derived from calcined clay and sugarcane bagasse ash incorporated with activated carbon for removal of heavy metals from aqueous media. Characterization of the Geopolymer, and activated carbon were done using XRF, XRD and FT-IR. Column adsorption using geopolymer/activated carbon filters was employed with Pb (II) and Cu (II) as the test heavy metal ions while varying concentration, filter thickness, and pH. Halo broad peaks were observed in the diffraction patterns at angle 2-theta between 20°- 34° in both geopolymer and activated carbon an indication that they were amorphous. Geopolymer: activated carbon ratio of 2:1, filter thickness of 4cm and pH range of 4-6.5 gave removal percentage of above 85% for both heavy metal ions. The experimental data fitted best in Freundlich adsorption isotherm with correlation coefficient (R2) of 0.854, 0.978 for Pb (II) ions, and Cu (II) respectively. Adsorption capacities of 0.028 mg/g and 0.015mg/g for Pb (II) ions, and Cu (II) ions were obtained respectively. The study shows that geopolymers/activated carbon filters have a potential of removing heavy metal ions from aqueous solution and hence can be applied for remediation of wastewater from industrial effluents.
Keywords: Activated carbon, Characterization, Geopolymers Filter, Hheavy metals, Wastewater
Cite this paper: D. M. Mwaura, H. M. Mbuvi, F. M. Maingi, Geopolymers Filters from Calcined Clay and Sugarcane Bagasse Blended with Activated Carbon for Removal of Selected Heavy Metals from Wastewater, American Journal of Chemistry, Vol. 12 No. 2, 2022, pp. 32-39. doi: 10.5923/j.chemistry.20221202.02.
Article Outline
1. Introduction
- Sources of heavy metal pollution are mining waste and industrial wastewater. When these waters are not taken care of, they lead to heavy metal pollution [1]. According to Ndeda and Manohar [2], the Nairobi dam is highly polluted with Pb(II), Cd(II), Cu(II), and Ni(II) ions hence the water is unsuitable for domestic and agricultural use. Muiruri, et al. [3], also concluded that there is bio-accumulation of heavy metals in fish at Athi-Galana, Sabaki tributaries in Kenya. Kaluli et al. [4] confirmed that irrigation of food crops with wastewater in Nairobi-Kenya increased the concentration of heavy metals such as lead and cadmium in food production to unsafe levels. Ravindra, et al. [5] clearly stated that the presence of heavy metals and their toxicity in the environment and human beings is a problem of global concern, and various ways of removing them should be studied. Adsorption of ingested lead may bring about chronic effects of lead poisoning hence causing constipation, colic, and anemia [6]. Extreme amounts of Cu (II) ions in the human body have been known to cause mucosal irritation, aquatic fauna, and central nervous system irritation followed by depression [5]. Geopolymers have been proven to be good adsorbents of heavy metal ions from solutions [7], [8]. Most researchers have used commercial raw materials in their geopolymerization process while Maingi et al. [9] used clay and rice husks ash but there is little information in the literature on the use of sugarcane bagasse ash. Maingi et al. [9] experienced clogging in his column adsorption and hence the need to remove clogging if the geopolymers have to be used for industrial wastewater management. This study aimed at developing a safe and easy way of removing heavy metals from an aqueous medium with minimal or no clogging. Therefore, the focus of this study was to fabricate geopolymer filters using calcined clay, sugarcane bagasse ash, and incorporating activated carbon to increase porosity and reduce clogging during the adsorption of heavy metal ions from an aqueous solution.
2. Material and Methods
2.1. Chemicals and Reagents
- The chemicals used were of analytical grade and obtained from Kobian Kenya. They included Pb(NO3)2 and Cu(NO3)2 for preparing synthetic wastewater. HCl was used to de-aluminate and remove iron from the sugarcane bagasse (SBA), and adjust the pH during the adsorption process. NaOH was used as an alkali-activator during geopolymerization process. KBr was used in FT-IR for characterization. ZnCl2 was used as an activator in the production of activated carbon.
2.2. Raw Materials
2.2.1. Clay as a Raw Material
- Clay was obtained locally at a clay working site in Kikuyu, Kenya. The clay was washed with distilled water and dried for ten days. Calcination was carried out in a furnace (Eisklo 120) at 670°C and then grounded to fine particles [10]. The powdered calcined clay obtained was used as a source of alumina and silica which are key ingredients for geopolymerization.
2.2.2. Sugarcane Bagasse Ash
- Sugarcane bagasse waste was obtained from sugar cane juice vendors in the Githurai market, Kenya. The sugarcane bagasse was cleaned using deionized distilled water to remove earthy materials and then air-dried for one week. Pyrolysis of the sugarcane bagasse was carried out in a furnace at 1000°C for 4 hours to obtain ash [11]. 5g of sugar cane bagasse ash (SBA) was placed in a 1000 mL Erlenmeyer flask and 50 mL of 1M HCl was added while stirring for 2 hours at room temperature to de-aluminate and remove iron present in SBA. The mixture was then filtered and the residue washed with distilled water to remove metal ions. The residue was then dried for 24 hours at 40°C [12]. The SBA obtained was used as a source of extra silica required in the geopolymerization process.
2.3. Synthesis of Geopolymer
- 8 M NaOH solutions were added to SBA in in the ratio of 5L: 1Kg and the mixture was stirred for 15 minutes. 50 g of the powdered calcined clay was then added to the mixture. The mixture was stirred for 5 hours using a magnetic stirrer [9]. The paste obtained was poured into ceramic crucibles and cured in an oven at 100°C for 10 hours for polycondensation to occur [13]. The cured geopolymer paste was then removed from the mold, washed with distilled water to remove excess alkali, and placed in an oven to dry at 150°C for 12 hours.
2.4. Preparation of Activated Carbon
- 1200 g of sawdust was properly washed to remove earthy materials and then air-dried for 5 days in the open to remove loosely bound water. The sawdust was then, placed in an oven and dried at 110°C for 10 hours before crushing and grinding [14]. The impregnating ratio of sawdust and zinc chloride of 1:1 was used at a temperature of 600–800°C for 3 hours to yield activated carbon with good porosity and surface area [15]. The activated char was leached with 1M HCl solution for 24 hours to remove excess activator, then washed thoroughly with distilled water, and dried at 80°C for 6 hours [16].
2.5. Characterization of Geopolymer and Activated Carbon
- The characterization was carried out using FT-IR (IR tracer) and XRD (D8 Advanced, BRUKER AXS). FT-IR was used for functional group analysis. A fine powdered sample was ground using a mortar and pestle. KBr was mixed with the powder at a ratio of 1: 20. The mixture was then compressed in a sample holder of the infrared machine and analyzed in varying wavelengths. XRD was used to identify the mineralogical composition of the geopolymer, phase compositions, and crystallinity hence the structural properties of the geopolymer using the model (Bruker AXS D8 Advance). Continuous 2-theta scans in the locked coupled mode were used for measurement with a tube of Cu-Ka radiation (lKa=1.5406Å) and a Lynx Eye position-sensitive detector.
2.6. Adsorption Experiments
2.6.1. Effects of the Ratio of Geopolymer to Activated Carbon on Heavy Metal Ions Removal
- Geopolymer was mixed thoroughly with activated carbon in a ratio ranging from 2:1 to 1:2. The different ratios were used in fabricating column filters that were employed for column adsorption. 50 mL of 100 mg/L of Cu (II) and Pb (II) were added onto each column filter separately and allowed to seep through the column with aid of gravity. The adsorption experiments were carried out in triplicates. The concentration of residues metal ions in emerging solutions was determined using FASS.
2.6.2. Effects of Filter Thickness on Heavy Metal Ions Adsorption
- The filter thickness was varied from 6 cm to 15 cm for the adsorption of the heavy metal ions. The study was done in triplicates. 50 mL of 100 mg/L of Cu (II) and Pb (II) were added onto each column filter and allowed to seep through the column with aid of gravity. The residual metal ions concentration in the filtrate was analyzed using FASS.
2.6.3. Effects of pH on Heavy Metal Ions Adsorption
- Optimization of pH was done by varying the pH of the working solution of 100.mg/L of Pb (II) and Cu (II) from 2 to 6. The filtered solutions were analyzed for residual metal ions concentrations using FAAS at a wavelength of 283.3nm for Pb (II) ions, and 324.8nm for Cu (II) ions.
2.6.4. Effects of Initial Metal Ion Concentration on Adsorption
- The effect of initial metal ion concentration on the efficiency of the geopolymer filters was studied using 50mL of each metal ion with concentrations varied from (15, 30, 75, 100, and 150) mg/L. The mixtures were passed through the geopolymer filters with aid of gravitational force. The filtered solution was analyzed for residual metal ions using FASS.
2.7. Adsorption Isotherms
- Langmuir and Freundlich isotherms were used to determine the adsorption capacities of the geopolymer filters. Equation 1 shows the linearized Langmuir adsorption isotherm used [17].
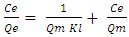 | (1) |
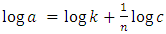 | (2) |
3. Results and Discussions
3.1. Chemical Composition of the Geopolymer Raw Materials
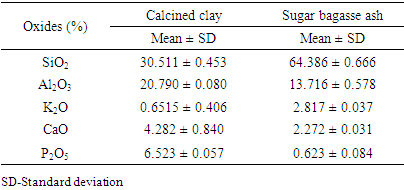
- X-ray fluorescence spectrometer was used in the determination of the chemical composition for the raw materials used. The main components of the calcined clays and sugar bagasse ash were oxides. Table 1 shows the mean% oxides obtained when calcined clay and SBA was analyzed using XRF.
|
3.2. Chemical Composition of the Geopolymer and Activated Carbon
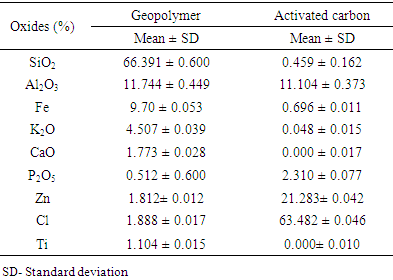
- The chemical composition of geopolymer and activated carbon used in fabrication of geopolymer filters is presented in table 2.
|
3.3. Characterization of Geopolymer and Activated Carbon Using FT-IR
- Fourier Transform Infra-Red analysis was carried out for spectroscopic analysis. The identification of functional groups in both geopolymer and activated carbon is critical for the adsorption of heavy metals. FT-IR absorption spectra were recorded in the wavelength range of 4600-150 cm-1, using FT-IR (IR tracer 100 Shimadzu). Potassium chloride was used to make the nujol mull to be analyzed.In the geopolymer, the spectra band at 989.50 cm-1 could be attributed to Si-O-Al and Si-O-Si asymmetrical vibrations. The band at 1451.46 cm-1 could be a result of O-C-O, formed from remaining unreacted activator and carbon (IV) oxide [21]. The peak at 1641.45 cm-1 corresponds to H-O-H bending vibrations, and at 3415.03 is related to –OH, H-O-H bending vibrations [21]. For activated carbon, the spectra band at 3415.03 is attributed to the stretching O-H bond in the phenolic, carboxylic functional group, and adsorbed water [22]. These functional groups obtained in both geopolymer and activated carbon is vital in the adsorption of positively charged metal ions through electrostatic attraction.
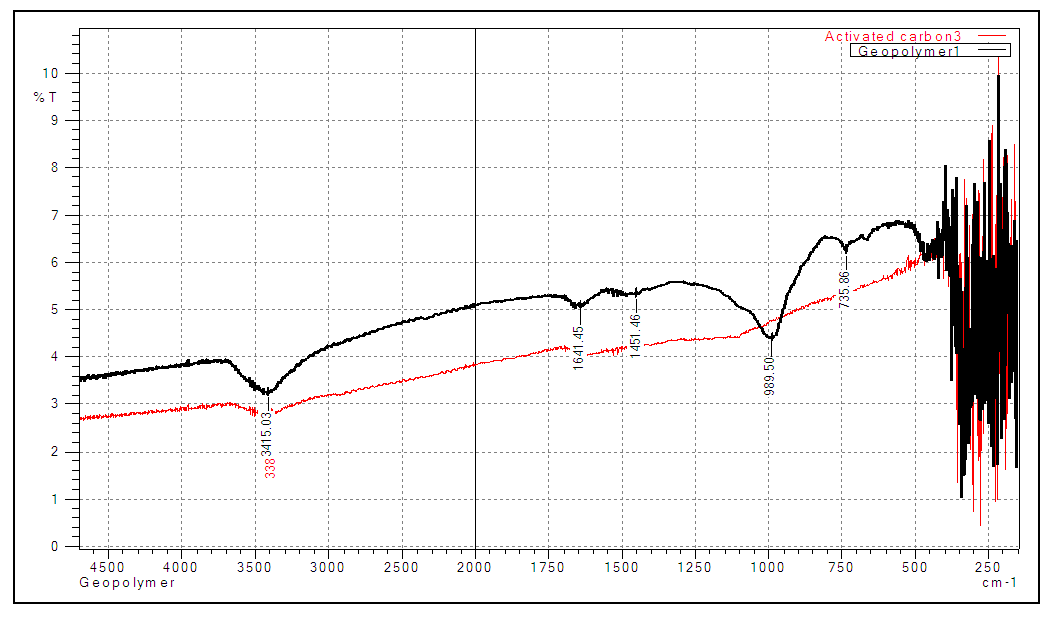 | Figure 1. FT-IR spectra for geopolymer, and activated carbon |
3.4. Characterization of Geopolymer, and Activated Carbon Using XRD
- X-ray diffraction was required to give details on whether the geopolymer and activated carbon were amorphous or crystalline. If the high percentage was crystalline it would indicate that the adsorption sites were fewer than expected but if it was amorphous it would illustrate that the adsorption site were more exposed [23]. Figure 2 shows the diffraction pattern of geopolymer.
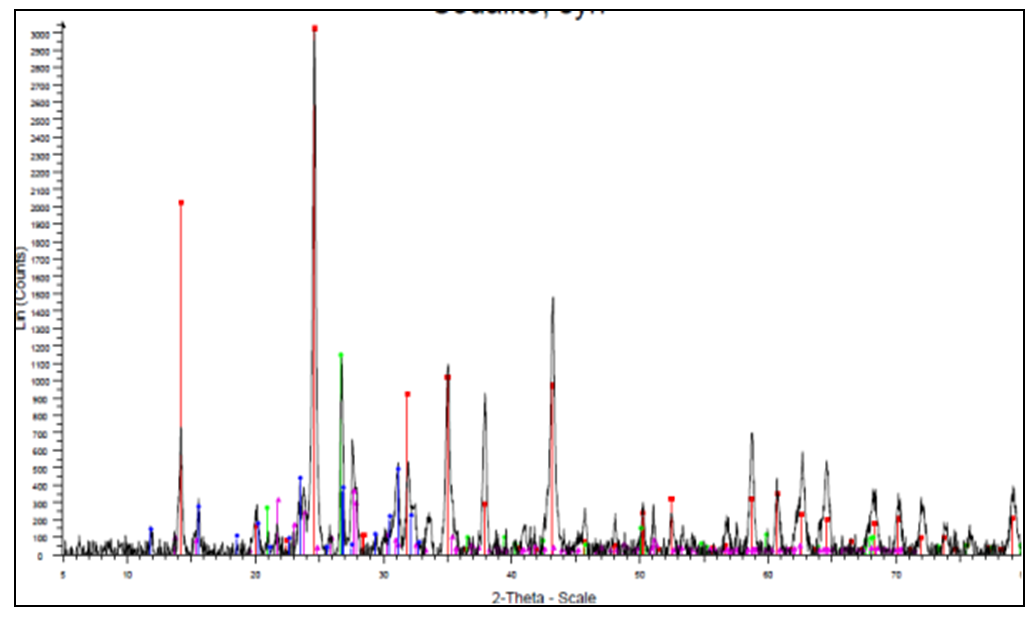 | Figure 2. Powder diffraction of the Geopolymer |
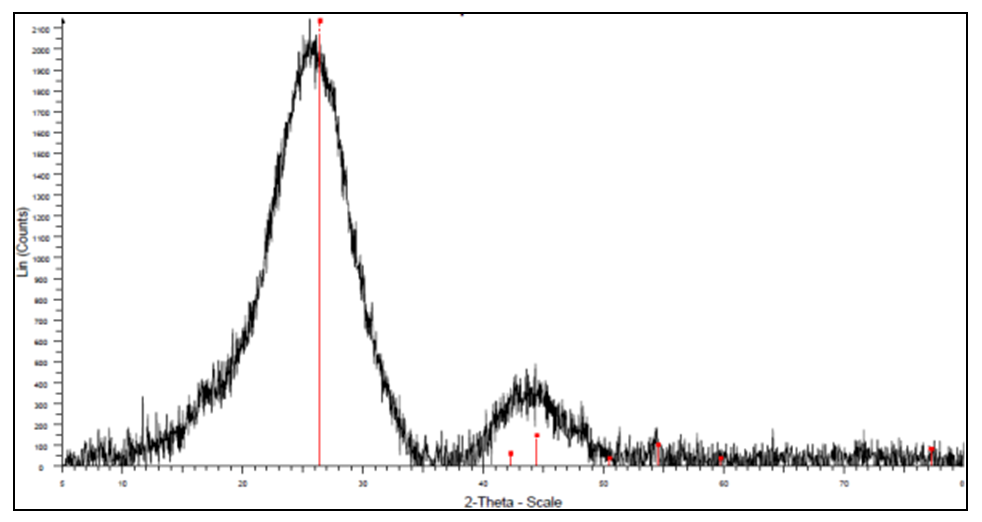 | Figure 3. Powder diffraction of the activated carbon |
3.5. Adsorption of Pb (II) and Cu (II) Ions Using the Geopolymer/ Activated Carbon Filters
3.5.1. Effects of Geopolymer: Activated Carbon Ratios on the Adsorption of Pb (II) Ions, and Cu (II) Ions
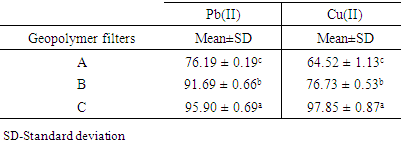
- Geopolymer: activated carbon ratio was studied using three filters, A, B, and C. Filters A, B, and C had a ratio of 1:2, 1:1, and 2:1 respectively. Table 3 shows the effect of varying the ratio of geopolymer: activated carbon on the adsorption of Pb (II), and Cu (II) ions from aqueous solutions.
|
3.5.2. Effects of Filter Thickness on the Adsorption of Pb (II) and Cu (II) Ions
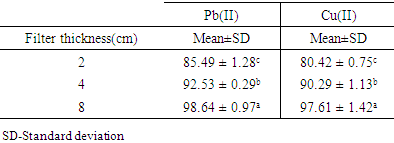
- Three different filter thicknesses of 2cm, 4cm, and 8cm were used. Table 4 shows the results of effect of varying filter thickness on adsorption of Pb (II) and Cu (II) ions from aqueous solutions.
|
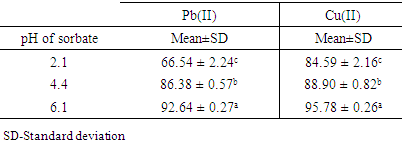
3.5.3. Effects of Initial pH on the Adsorption of Pb (II) and Cu (II) Ions
- Table 5 shows the effect of varying pH on the adsorption of Pb (II) and Cu (II) ions from synthetic wastewater when geopolymer: activated carbon ratio of 2:1 and filter thickness of 4 cm was used.
|
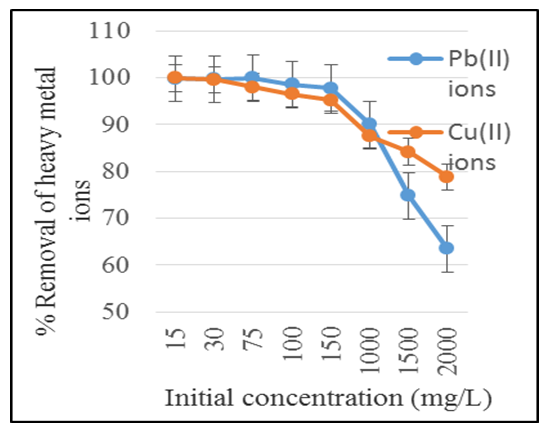
3.5.4. Effects of Initial Metal Ion Concentration on the Adsorption of Pb (II) and Cu (II) Ions
- The initial metal ion concentration of any solution is important since it indicate the concentration in which the adsorbent is most effective. It also indicates how much of the adsorbent is to be used or how much of the solution should be passed through the filter. Figure 4 shows the effect of initial concentration on the adsorption of Pb.(II) and Cu.(II) ions from aqueous solutions.
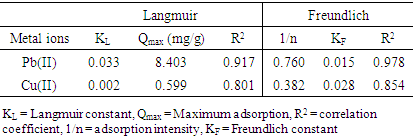
3.6. Equilibrium Studies Using Adsorption Isotherms
- The adsorption of Pb (II) and Cu (II) ions was studied using Langmuir and Freundlich adsorption isotherms. Table 6 shows the isotherm constants for adsorption of Pb (II) and Cu (II) respectively.
|
4. Conclusions
- FT-IR analysis showed the presence of functional groups in the geopolymer filter vital for the adsorption of heavy metals. Phase composition analysis using XRD showed that the geopolymer was crystalline and activated carbon was amorphous therefore mixing the two reduced the crystallinity level and hence increasing the adsorption process. The geopolymer: activated carbon ratio of 2:1 and filter thickness of 4cm gave the highest removal rate of the Pb (II) and Cu (II) ions. Experimental data fitted best in linearized Freundlich isotherm an indication that the adsorption occurred on a heterogonous surface. This study has shown that geopolymer filters derived from calcined clay, sugar bagasse, and activated carbon has high potential of being used as filters for the removal of heavy metals from an aqueous solution.
ACKNOWLEDGEMENTS
- The authors would like to express their sincere gratitude to Department of Chemistry Kenyatta University and Department of Science Technology and Engineering, Kibabii University for assistance offered during this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML