-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2022; 12(1): 18-21
doi:10.5923/j.chemistry.20221201.03
Received: Mar. 1, 2022; Accepted: Mar. 16, 2022; Published: Mar. 24, 2022

The Mechanism of the Oxido-degradation of the Cinchona Alkaloids
Francisco Sánchez-Viesca, Reina Gómez
Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico
Correspondence to: Francisco Sánchez-Viesca, Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The cinchona alkaloids, so important for malaria treatment, have been studied from different points of view such as chemical structure, biological properties and synthesis. However the reaction mechanisms involved in several oxidative degradations have not been advanced. In this communication we provide the electron flow that takes place in the reaction series with different oxidizers and substrates. The chemical deportment of both reagent and substrate has been taken into account: the dual function of chromic acid, that is, as nucleophile (chromate anion) and as electrophile (protonated chromium trioxide), as well as the successive reduction of Mn(VII) to Mn(V) and Mn(III), yielding finally Mn(IV) in manganese dioxide; also the reactivity of α-diketones to form a hydrate, a key intermediate for C─C fission to give a couple of carboxylic acids. The oxido-degradation by means of acidic potassium permanganate of the vinyl and carboxymethyl groups, present in quinuclidine and piperidine rings, has been traced to the end.
Keywords: Cinchonine, Cinchoninic acid, Cinchotenine, Meroquinene, Piperidine, Quinuclidine
Cite this paper: Francisco Sánchez-Viesca, Reina Gómez, The Mechanism of the Oxido-degradation of the Cinchona Alkaloids, American Journal of Chemistry, Vol. 12 No. 1, 2022, pp. 18-21. doi: 10.5923/j.chemistry.20221201.03.
1. Introduction
- Malaria is caused by three species of protozoa, Plasmodium vivax, tertian parasite, Plasmodium malaria, quartan parasite, and Plasmodium falciparum, malignant tertian malaria, frequently results in fatalities unless a suitable drug is administered properly.The natural cinchona alkaloids have antimalaria activity, quinine and cinchonine are found in Cinchona officinalis. Cinchonine is more efficient than quinine, it lacks a methoxy group present in quinine. One of the best sources of cinchonine is Cinchona micrantha bark.Although the structures of these compounds and the degradation products are known, the mechanism of the oxidations carried out has not been advanced. In this communication we provide the electron flow in the reaction series that occurs during different oxido-degradation processes.This paper is a follow up of our studies on reaction mechanism [1-5].
2. Antecedents
- The nomenclature of the cinchona alkaloids is based in the ruban structure, Figure 1, from Rubiaceae [6], since Cinchona is a genus in that family.
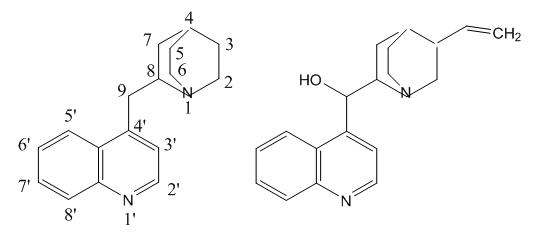 | Figure 1. Ruban and cinchonine structures |
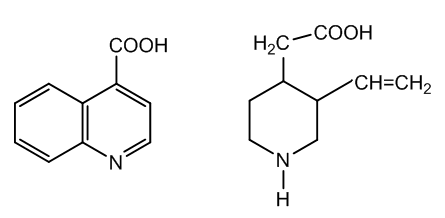 | Figure 2. Cinchoninic acid and meroquinene |
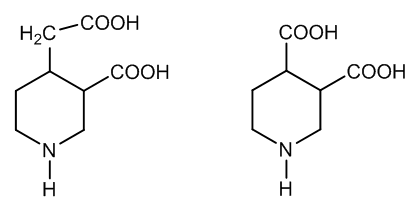 | Figure 3. Cincholoiponic acid and loiponic acid |
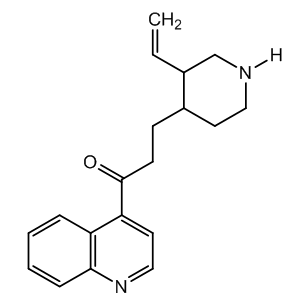 | Figure 4. Cinchotoxine (Cinchonicine) |
3. Discussion
- The oxidative degradation of cinchonine to cinchoninic acid and meroquinene goes through the first oxidation product, cinchoninone. The enolic form of this ketone reacts with protonated chromic trioxide, this electrophile coming from acid catalysed dehydration of chromic acid.Acid hydrolysis of the organometallic intermediate yields the reduced H2CrO3 with Cr(IV) and a reactive carbinol-amine which forms a diketone and a secondary amine via ring fission, Figure 5.
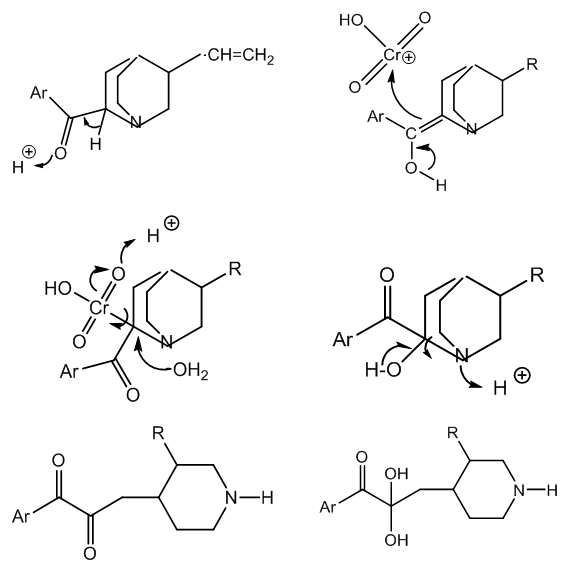 | Figure 5. From cinchoninone to the hydrated diketone after quinuclidine ring opening |
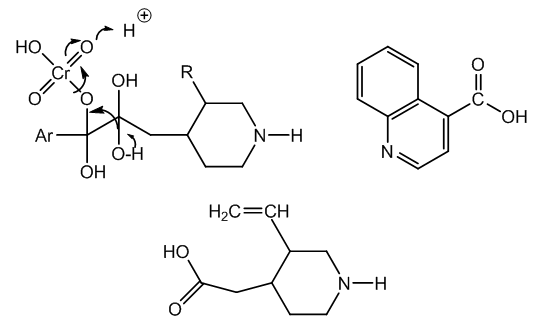 | Figure 6. Formation of H2CrO3, cichoninic acid and meroquinene |
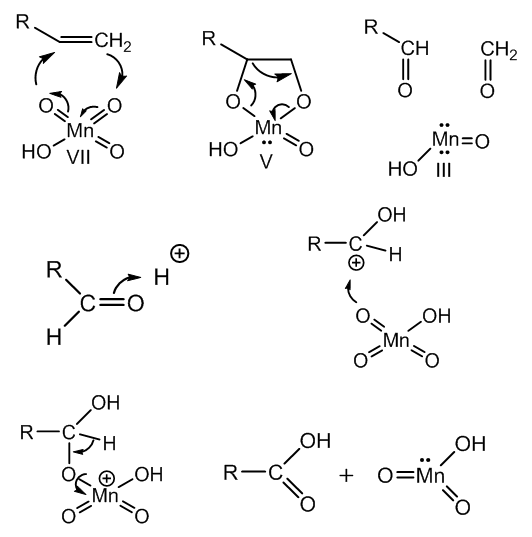 | Figure 7. Oxidation of the vinyl group in meroquinene to cincholoiponic acid |
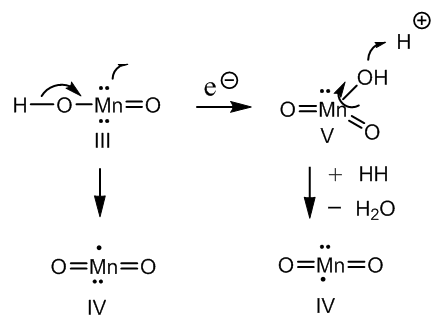 | Figure 8. Formation of manganese dioxide from Mn(III) and Mn(V) intermediates |
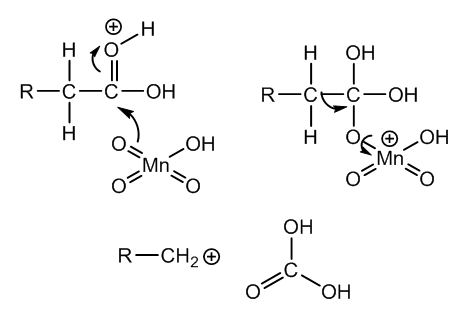 | Figure 9. First steps of the degradation of cincholoiponic acid to loiponic acid via elimination of carbonic acid |
4. Conclusions
- The oxido-degradation of cinchonine by means of chromic acid goes through cinchoninone. An organometallic intermediate is formed via enolization and acid hydrolysis yields an unstable carbinolamine. A vicinal diketone is formed with concomitant ring fission of the quinuclidine moiety The aliphatic ketone is hydrated and the aromatic ketone adds a molecule of chromic acid. Protonation of the chromic ester gives raise a concerted mechanism affording cinchoninic acid and meroquinene.KMnO4/H2SO4 oxidation of meroquinene in two experimental steps forms cincholoiponic and loiponic acids. The first acid is produced by interaction with a vinyl group: a cyclic Mn(V) intermediate is cleaved by reduction to Mn(III) and C─C fission. Two aldehydes are produced whose further oxidation gives cincholoiponic acid and formic acid.Loiponic acid results by shortening of the carboxymethyl group to carboxyl. Formation of an oxonium ion permits reaction with permanganic acid. There is elimination of HMnO3 and carbonic acid, a primary carbocation remains whose reaction with water and two further oxidations affords a carboxyl.This way the oxido degradations of cinchonine, representative cinchona alkaloid, have been studied.
ACKNOWLEDGEMENTS
- Thanks are given to Martha Berros for support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML